npj Breast Cancer ( IF 6.5 ) Pub Date : 2018-11-14 , DOI: 10.1038/s41523-018-0090-6 Charles E. Geyer , Gong Tang , Eleftherios P. Mamounas , Priya Rastogi , Soonmyung Paik , Steven Shak , Frederick L. Baehner , Michael Crager , D. Lawrence Wickerham , Joseph P. Costantino , Norman Wolmark
|
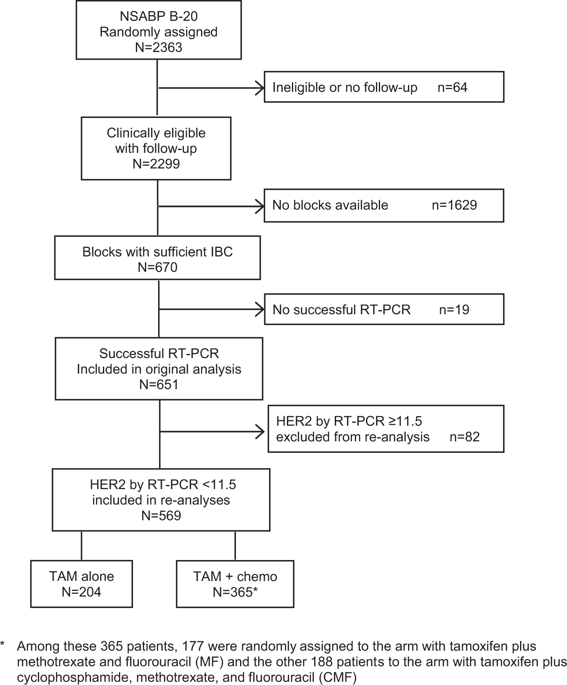
The NSABP B-20 prospective-retrospective study of the 21-gene Oncotype DX Breast Cancer Recurrence Score® test predicted benefit from addition of chemotherapy to tamoxifen in node-negative, estrogen-receptor positive breast cancer when recurrence score (RS) was ≥31. HER2 is a component of the RS algorithm with a positive coefficient and contributes to higher RS values. Accrual to B-20 occurred prior to routine testing for HER2, so questions have arisen regarding assay performance if HER2-positive patients were identified and excluded. We report an exploratory reanalysis of the B-20, 21-gene study following exclusion of such patients. Patients were considered HER2 positive if quantitative RT-PCR for HER2 was ≥11.5 units, and excluded from re-analyses performed using the original cutoffs: <18, 18–30, ≥31, and the TAILORx cutoffs: <11, 11–25, >25. The endpoint remained distant recurrence-free interval (DRFI) as in the original study. Distribution was estimated via the Kaplan–Meier method and compared via log-rank test. Multivariate Cox proportional hazards models estimated chemotherapy benefit in each group. In the RS < 18 and 18–30 groups, 1.7 and 6.7% were HER2 positive. In the RS ≥ 31 group, 41% were HER2 positive. Exclusion resulted in fewer events, with loss of significance for benefit from chemotherapy in the overall HER2-negative cohort (log-rank P = 0.06), but substantial benefit from chemotherapy remained in the RS ≥ 31 cohort (HR = 0.18; 95% CI: 0.07–0.47) and the RS > 25 cohort (HR = 0.28; 95% CI: 0.12–0.64). No benefit from chemotherapy was evident in the other RS groups. Following exclusion of HER2-positive patients based on RT-PCR expression, substantial benefit of chemotherapy remained for RS ≥ 31 as originally employed, and with RS > 25 employed in TAILORx.
中文翻译:

21基因测定可预测HER2阴性乳腺癌的化疗获益
对21基因Oncotype DX乳腺癌复发评分®试验的NSABP B-20前瞻性研究预测,当复发评分(RS)≥31时,他莫昔芬对淋巴结阴性,雌激素受体阳性的乳腺癌患者增加化疗的获益。 。HER2是具有正系数的RS算法的组成部分,有助于提高RS值。对B-20的应计发生在常规检测HER2之前,因此,如果鉴定并排除了HER2阳性患者,则有关测定性能的问题就出现了。我们报告了排除此类患者后对B-20、21基因研究进行的探索性重新分析。如果HER2的定量RT-PCR≥11.5个单位,则患者被视为HER2阳性,并且被排除在使用原始截止值:<18、18–30,≥31和TAILORx截止值:<11、11–25进行的重新分析中,> 25。与原始研究一样,终点仍保持遥远的无复发间隔(DRFI)。分布通过Kaplan-Meier方法估算,并通过对数秩检验进行比较。多元Cox比例风险模型估计了每组的化疗获益。在RS <18和18–30组中,HER2阳性为1.7和6.7%。在RS≥31组中,41%的患者为HER2阳性。排除导致更少的事件,并且在整个HER2阴性队列中对化疗获益的重要性丧失(对数秩 7%为HER2阳性。在RS≥31组中,41%的患者为HER2阳性。排除导致更少的事件,并且在整个HER2阴性队列中对化疗获益的重要性丧失(对数秩 7%为HER2阳性。在RS≥31组中,41%的患者为HER2阳性。排除导致更少的事件,并且在整个HER2阴性队列中对化疗获益的重要性丧失(对数秩P = 0.06),但RS≥31组(HR = 0.18; 95%CI:0.07–0.47)和RS> 25组(HR = 0.28; 95%CI:0.12-0.64)仍可从化疗中获益。在其他RS组中,没有从化疗中获益的证据。根据RT-PCR表达排除HER2阳性的患者后,对于RS≥31,如最初采用的方法,以及在TAILORx中采用RS> 25的方法,化疗仍具有实质性的获益。











































 京公网安备 11010802027423号
京公网安备 11010802027423号