NeuroToxicology ( IF 3.4 ) Pub Date : 2018-11-05 , DOI: 10.1016/j.neuro.2018.11.001 Elaine Wan Ling Chan , Sangeetha Krishnansamy , Cindy Wong , Sook Yee Gan
|
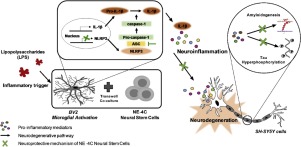
The cognitive impairment caused by Alzheimer’s disease (AD) is associated with beta-amyloid (Aβ) and tau proteins, and is accompanied by inflammation. Recently, a novel inflammasome signaling pathway has been uncovered. Inflammasomes are implicated in the execution of inflammatory responses and pyroptotic death leading to neurodegeneration. Thus, the inflammasome signaling pathway could be a potential therapeutic target for AD. Neural stem cells (NSCs) are multipotent cells that can self-renew and differentiate into distinct neural cells. NSC therapy has been considered to be a promising therapeutic approach in protecting the central nervous system and restoring it following damage. However, the mechanisms involved remain unclear. The aims of this study were to investigate the protective effects of NE4C neural stem cells against microglia-mediated neurotoxicity and to explore molecular mechanisms mediating their actions. NE4C decreased the levels of caspase-1 and IL-1β, and attenuated the level of the NLRP3 inflammasome and its associated protein adapter, apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC) in LPS-stimulated BV2 microglial cells, possibly by regulating the phosphorylation of p38α MAPK. The conditioned media obtained from co-culture of LPS-stimulated BV2 and NE4C cells exhibited protective effects on SH-SY5Y cells against microglia-mediated neurotoxicity; this was associated with an attenuation of tau phosphorylation and amyloidogenesis and accompanied by down-regulation of GSK-3β and p38α MAPK signalling pathways. In conclusion, the present study suggested that NSC therapy could be a potential strategy against microglia-mediated neurotoxicity. NSCs regulate NLRP3 activation and IL-1β secretion, which are critical in the initiation of the inflammatory responses, hence preventing the release of neurotoxic pro-inflammatory factors by microglia. This eventually reduces tau hyperphosphylation and amyloidogenesis, possibly through the regulation of GSK-3β and p38α MAPK signalling pathways, and thus protects SH-SY5Y cells against microglia-mediated neurotoxicity.
中文翻译:

NLRP3炎性小体通过抑制tau过度磷酸化和淀粉样蛋白生成参与神经干细胞对抗小胶质细胞介导的SH-SY5Y细胞毒性的神经保护机制。
由阿尔茨海默氏病(AD)引起的认知障碍与β-淀粉样蛋白(Aβ)和tau蛋白有关,并伴有炎症。最近,已经发现了新型的炎性体信号传导途径。炎性小体与炎症反应的执行和导致神经变性的焦磷酸化死亡有关。因此,炎性体信号通路可能是AD的潜在治疗靶标。神经干细胞(NSC)是能自我更新并分化为不同神经细胞的多能细胞。NSC治疗被认为是保护中枢神经系统并在受损后恢复其功能的一种有前途的治疗方法。但是,所涉及的机制仍不清楚。这项研究的目的是调查NE4C神经干细胞对小胶质细胞介导的神经毒性的保护作用,并探讨介导其作用的分子机制。NE4C降低了caspase-1和IL-1β的水平,并减弱了LPRP刺激的BV2中含有C末端caspase募集域(ASC)的凋亡相关斑点样蛋白NLRP3炎性小体及其相关蛋白衔接子的水平。小胶质细胞,可能是通过调节p38αMAPK的磷酸化来实现的。从LPS刺激的BV2和NE4C细胞共培养获得的条件培养基对SH-SY5Y细胞具有抗小胶质细胞介导的神经毒性的保护作用。这与tau磷酸化和淀粉样蛋白生成的减弱有关,并伴有GSK-3β和p38αMAPK信号通路的下调。总之,本研究表明,NSC治疗可能是对抗小胶质细胞介导的神经毒性的潜在策略。NSC调节NLRP3激活和IL-1β分泌,这在引发炎症反应中至关重要,因此可防止小胶质细胞释放神经毒性促炎因子。这最终可能通过调节GSK-3β和p38αMAPK信号通路来减少tau的高磷酸化和淀粉样蛋白生成,从而保护SH-SY5Y细胞免受小胶质细胞介导的神经毒性。因此可以防止小胶质细胞释放神经毒性促炎因子。这最终可能通过调节GSK-3β和p38αMAPK信号通路来减少tau的高磷酸化和淀粉样蛋白生成,从而保护SH-SY5Y细胞免受小胶质细胞介导的神经毒性。因此可以防止小胶质细胞释放神经毒性促炎因子。这最终可能通过调节GSK-3β和p38αMAPK信号通路来减少tau的高磷酸化和淀粉样蛋白生成,从而保护SH-SY5Y细胞免受小胶质细胞介导的神经毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号