Catalysis Today ( IF 5.2 ) Pub Date : 2018-10-30 , DOI: 10.1016/j.cattod.2018.10.038 G. Bonura , A.A. Khassin , T.M. Yurieva , C. Cannilla , F. Frusteri , L. Frusteri
|
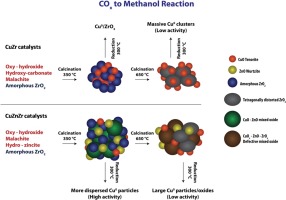
A series of coprecipitated binary Cu-ZrO2 catalysts was found to show an interesting activity–selectivity pattern during methanol synthesis from catalytic hydrogenation of carbon oxides (PR, 20–30 atm; TR, 200–240 °C). The effects of various pre–treatments as well as the copper/zirconia ratio on the structural and chemical properties of these samples were examined. The isoconversional Ozawa-Flynn-Wall method was applied to study the reduction behaviour, while the best fit modelling was used to establish the plausible mechanism of copper reduction. The extent of methanol formation rate was found to be dependent on the structure formed upon catalyst reduction, both in CO and in CO2 hydrogenation conditions. The pre–calcination of the sample at a temperature as high as 650 °C negatively affected the methanol formation rate under CO hydrogenation conditions, while under the same activation treatment an increased specific activity was observed in CO2 hydrogenation conditions, although with a minor methanol selectivity, since the rate of the WGS reaction was stronger enhanced. The incorporation of Zn into the catalyst formulation resulted in a visible increasing of the methanol formation rate, owing to the formation of a copper–zinc mixed oxide during calcination, which leads to higher metal dispersion also depressing the methane formation rate.
中文翻译:

碳氧化物催化加氢制甲醇中含Zr的混合氧化物中铜还原动力学的结构控制
一系列共沉淀的二进制的Cu-的ZrO 2催化剂被发现显示出甲醇合成期间从碳氧化物的催化氢化一个令人感兴趣的活性,选择性图案(P - [R,20-30大气压; Ť - [R,200-240℃)。研究了各种预处理以及铜/氧化锆比对这些样品的结构和化学性质的影响。采用等转化Ozawa-Flynn-Wall方法研究还原行为,同时使用最佳拟合模型建立合理的铜还原机理。发现甲醇形成速率的程度取决于在CO和CO 2中催化剂还原时形成的结构。氢化条件。样品在温度高达650℃下的预焙烧CO氢化条件下的甲醇形成率的负面影响,而同样的活化处理下在CO中观察到的增加的比活性2氢化条件,尽管具有轻微的甲醇选择性,因为WGS反应的速率更强。由于在煅烧过程中形成了铜锌混合氧化物,因此在催化剂配方中掺入Zn导致甲醇的形成速率明显增加,这导致较高的金属分散度也降低了甲烷的形成速率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号