当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Insights into the Substrate Promiscuity of a Laboratory-Evolved Peroxygenase
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-10-30 00:00:00 , DOI: 10.1021/acschembio.8b00500 Mercedes Ramirez-Escudero 1 , Patricia Molina-Espeja 2 , Patricia Gomez de Santos 2 , Martin Hofrichter 3 , Julia Sanz-Aparicio 1 , Miguel Alcalde 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-10-30 00:00:00 , DOI: 10.1021/acschembio.8b00500 Mercedes Ramirez-Escudero 1 , Patricia Molina-Espeja 2 , Patricia Gomez de Santos 2 , Martin Hofrichter 3 , Julia Sanz-Aparicio 1 , Miguel Alcalde 2
Affiliation
|
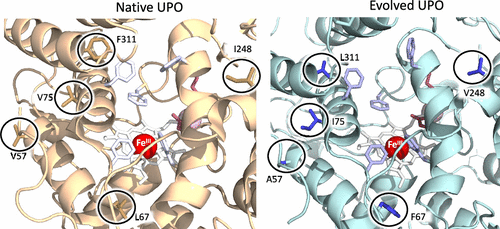
Because of their minimal requirements, substrate promiscuity and product selectivity, fungal peroxygenases are now considered to be the jewel in the crown of C–H oxyfunctionalization biocatalysts. In this work, the crystal structure of the first laboratory-evolved peroxygenase expressed by yeast was determined at a resolution of 1.5 Å. Notable differences were detected between the evolved and native peroxygenase from Agrocybe aegerita, including the presence of a full N-terminus and a broader heme access channel due to the mutations that accumulated through directed evolution. Further mutagenesis and soaking experiments with a palette of peroxygenative and peroxidative substrates suggested dynamic trafficking through the heme channel as the main driving force for the exceptional substrate promiscuity of peroxygenase. Accordingly, this study provides the first structural evidence at an atomic level regarding the mode of substrate binding for this versatile biocatalyst, which is discussed within a biological and chemical context.
中文翻译:

对实验室进化的过氧合酶底物混杂的结构见解
由于它们的最低要求,底物混杂和产物选择性,真菌过氧化酶现在被认为是C–H氧官能化生物催化剂中的佼佼者。在这项工作中,由酵母表达的第一个实验室进化的过氧合酶的晶体结构被确定为分辨率为1.5。在Agrocybe aegerita的进化过氧化物酶和天然过氧化物酶之间发现了显着差异,包括完整的N端和更宽的血红素访问通道,这归因于通过定向进化积累的突变。用过氧合和过氧化底物的调色板进行的进一步诱变和浸泡实验表明,通过血红素通道的动态运输是过氧化酶异常底物混杂的主要驱动力。因此,该研究在原子水平上提供了关于这种多功能生物催化剂的底物结合方式的第一个结构证据,这在生物学和化学背景下进行了讨论。
更新日期:2018-10-30
中文翻译:

对实验室进化的过氧合酶底物混杂的结构见解
由于它们的最低要求,底物混杂和产物选择性,真菌过氧化酶现在被认为是C–H氧官能化生物催化剂中的佼佼者。在这项工作中,由酵母表达的第一个实验室进化的过氧合酶的晶体结构被确定为分辨率为1.5。在Agrocybe aegerita的进化过氧化物酶和天然过氧化物酶之间发现了显着差异,包括完整的N端和更宽的血红素访问通道,这归因于通过定向进化积累的突变。用过氧合和过氧化底物的调色板进行的进一步诱变和浸泡实验表明,通过血红素通道的动态运输是过氧化酶异常底物混杂的主要驱动力。因此,该研究在原子水平上提供了关于这种多功能生物催化剂的底物结合方式的第一个结构证据,这在生物学和化学背景下进行了讨论。











































 京公网安备 11010802027423号
京公网安备 11010802027423号