当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Functional Studies of a gem-Dimethylating Methyltransferase from a trans-Acyltransferase Assembly Line
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-10-29 00:00:00 , DOI: 10.1021/acschembio.8b00733 Jessica L. Meinke 1 , M. Rachel Mehaffey 2 , Drew T. Wagner 1 , Ningze Sun 1 , Zhicheng Zhang 2 , Jennifer S. Brodbelt 2 , Adrian T. Keatinge-Clay 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-10-29 00:00:00 , DOI: 10.1021/acschembio.8b00733 Jessica L. Meinke 1 , M. Rachel Mehaffey 2 , Drew T. Wagner 1 , Ningze Sun 1 , Zhicheng Zhang 2 , Jennifer S. Brodbelt 2 , Adrian T. Keatinge-Clay 1
Affiliation
|
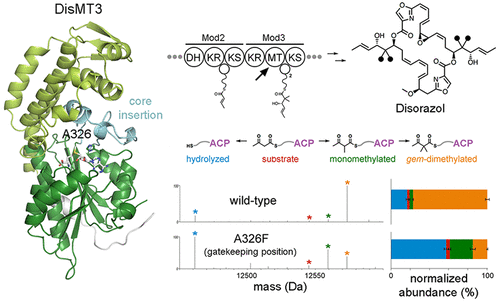
The methyl substituents in products of trans-acyltransferase assembly lines are usually incorporated by S-adenosyl-methionine (SAM)-dependent methyltransferase (MT) domains. The gem-dimethyl moieties within the polyketide disorazol are installed through the iterative action of an MT in the third module of its assembly line. The 1.75-Å-resolution crystal structure of this MT helps elucidate how it catalyzes the addition of two methyl groups. Activity assays of point mutants on β-ketoacyl chains linked to an acyl carrier protein and N-acetylcysteamine provide additional insights into the roles of active site residues. The replacement of an alanine with a phenylalanine at an apparent gatekeeping position resulted in more monomethylation than dimethylation. MTs may form an interface with ketoreductases (KRs) and even mediate the docking of trans-acyltransferase assembly line polypeptides through this association.
中文翻译:

反式-酰基转移酶组装生产线的宝石-二甲基化甲基转移酶的结构和功能研究
反式-酰基转移酶装配线产物中的甲基取代基通常通过S-腺苷甲硫氨酸(SAM)依赖性甲基转移酶(MT)域掺入。通过MT的迭代作用,将聚酮化合物二甲唑中的宝石-二甲基部分安装在其装配线的第三个模块中。该MT的1.75-Å分辨率晶体结构有助于阐明其如何催化两个甲基的加成。与酰基载体蛋白和N连接的β-酮酰基链上的点突变体的活性测定-乙酰半胱胺可提供有关活性位点残基作用的更多见解。在明显的看门位置将丙氨酸替换为苯丙氨酸比二甲基化产生更多的单甲基化。MT可以与酮还原酶(KR)形成界面,甚至通过该缔合介导反式酰基转移酶组装线多肽的对接。
更新日期:2018-10-29
中文翻译:

反式-酰基转移酶组装生产线的宝石-二甲基化甲基转移酶的结构和功能研究
反式-酰基转移酶装配线产物中的甲基取代基通常通过S-腺苷甲硫氨酸(SAM)依赖性甲基转移酶(MT)域掺入。通过MT的迭代作用,将聚酮化合物二甲唑中的宝石-二甲基部分安装在其装配线的第三个模块中。该MT的1.75-Å分辨率晶体结构有助于阐明其如何催化两个甲基的加成。与酰基载体蛋白和N连接的β-酮酰基链上的点突变体的活性测定-乙酰半胱胺可提供有关活性位点残基作用的更多见解。在明显的看门位置将丙氨酸替换为苯丙氨酸比二甲基化产生更多的单甲基化。MT可以与酮还原酶(KR)形成界面,甚至通过该缔合介导反式酰基转移酶组装线多肽的对接。









































 京公网安备 11010802027423号
京公网安备 11010802027423号