Nature Nanotechnology ( IF 38.1 ) Pub Date : 2018-10-22 , DOI: 10.1038/s41565-018-0273-1 Janos Szebeni 1, 2, 3 , Dmitri Simberg 4 , África González-Fernández 5 , Yechezkel Barenholz 6 , Marina A Dobrovolskaia 7
|
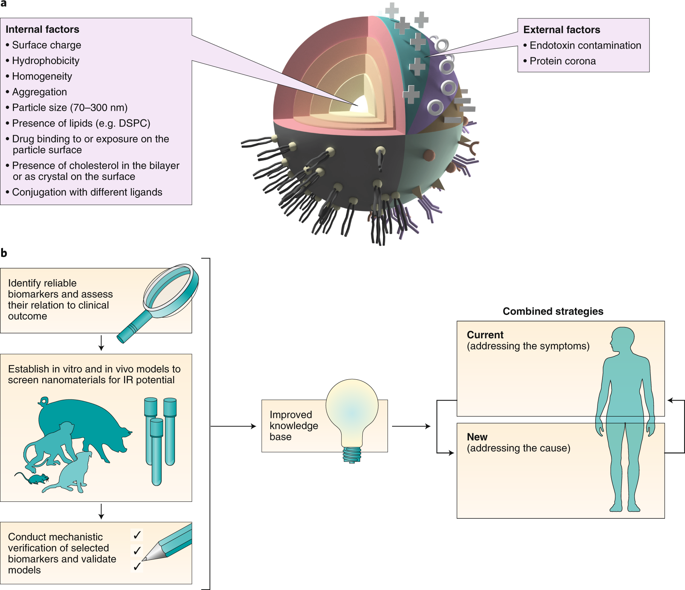
Infusion reactions (IRs) are complex, immune-mediated side effects that mainly occur within minutes to hours of receiving a therapeutic dose of intravenously administered pharmaceutical products. These products are diverse and include both traditional pharmaceuticals (for example biological agents and small molecules) and new ones (for example nanotechnology-based products). Although IRs are not unique to nanomedicines, they represent a hurdle for the translation of nanotechnology-based drug products. This Perspective offers a big picture of the pharmaceutical field and examines current understanding of mechanisms responsible for IRs to nanomedicines. We outline outstanding questions, review currently available experimental evidence to provide some answers and highlight the gaps. We review advantages and limitations of the in vitro tests and animal models used for studying IRs to nanomedicines. Finally, we propose a roadmap to improve current understanding, and we recommend a strategy for overcoming the problem.
中文翻译:

克服纳米药物输注反应的路线图和策略
输注反应(IR)是复杂的、免疫介导的副作用,主要发生在接受治疗剂量的静脉注射药品后几分钟到几小时内。这些产品多种多样,包括传统药物(例如生物制剂和小分子)和新药物(例如基于纳米技术的产品)。尽管 IR 并不是纳米药物所独有的,但它们对基于纳米技术的药物产品的转化构成了障碍。本视角提供了制药领域的全景图,并探讨了目前对纳米药物 IR 机制的理解。我们概述了悬而未决的问题,回顾了当前可用的实验证据,以提供一些答案并强调差距。我们回顾了用于研究纳米药物 IR 的体外试验和动物模型的优点和局限性。最后,我们提出了一个路线图来提高当前的理解,并提出了解决该问题的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号