Biochimie ( IF 3.3 ) Pub Date : 2018-10-13 , DOI: 10.1016/j.biochi.2018.10.005 Uris Ros , Gustavo P.B. Carretero , Joana Paulino , Edson Crusca , Fabiola Pazos , Eduardo M. Cilli , Maria E. Lanio , Shirley Schreier , Carlos Alvarez
|
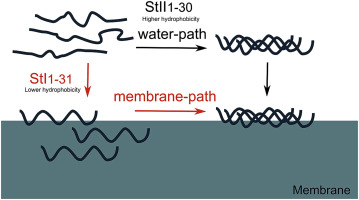
Sticholysin I and II (Sts: St I and St II) are proteins of biomedical interest that form pores upon the insertion of their N-terminus in the plasma membrane. Peptides spanning the N-terminal residues of StI (StI1-31) or StII (StII1-30) can mimic the permeabilizing ability of these toxins, emerging as candidates to rationalize their potential biomedical applications. These peptides have different activities that correlate with their hydrophobicity. However, it is not clear how this property contributes to peptide folding in solution or upon binding to membranes. Here we compared the conformational properties of these peptides and shorter versions lacking the hydrophobic segment 1–11 of StI (StI12-31) or 1–10 of StII (StII11-30). Folding of peptides was assessed in solution and in membrane mimetic systems and related with their ability to bind to membranes and to permeabilize lipid vesicles. Our results suggest that the differences in activity among peptides could be ascribed to their different folding propensity and different membrane binding properties. In solution, StII1-30 tends to acquire α-helical conformation coexisting with self-associated structures, while StI1-31 remains structureless. Both peptides fold as α-helix in membrane; but StII1-30 also self-associates in the lipid environment, a process that is favored by its higher affinity for membrane. We stress the contribution of the non-polar/polar balance of the 1–10 amino acid sequence of the peptides as a determining factor for different self-association capabilities. Such difference in hydrophobicity seems to determine the molecular path of peptides folding upon binding to membranes, with an impact in their permeabilizing activity. This study contributes to a better understanding of the molecular mechanisms underlying the permeabilizing activity of Sts N-terminal derived peptides, with connotation for the exploitation of these small molecules as alternative of the full-length toxins in clinical settings.
中文翻译:

膜中的自缔合和折叠决定了溶血素溶解片段中肽的作用方式
Sticholysin I和II(Sts:St I和St II)是具有生物医学意义的蛋白质,它们的N末端插入质膜后会形成孔。跨越StI(StI 1-31)或StII(StII 1-30)N末端残基的肽可以模拟这些毒素的通透能力,从而成为合理化其潜在生物医学应用的候选者。这些肽具有与其疏水性相关的不同活性。但是,尚不清楚该性质如何促进肽在溶液中或与膜结合时的折叠。在这里,我们比较了这些肽的构象特性和缺少StI疏水片段1-11(StI 12-31)或StII 1-10(StII 11-30)的短肽的构象特性)。在溶液和膜模拟系统中评估了肽的折叠,并与它们结合膜和透化脂质囊泡的能力有关。我们的结果表明,肽之间活性的差异可以归因于其不同的折叠倾向和不同的膜结合特性。在溶液中,StII 1-30倾向于获得与自缔合结构共存的α-螺旋构象,而StI 1-31则保持无结构。两种肽都在膜中折叠成α-螺旋。但是StII 1-30在脂质环境中也会自缔合,这一过程因其对膜的更高亲和力而受到青睐。我们强调肽的1-10个氨基酸序列的非极性/极性平衡的贡献,作为不同自我缔合能力的决定因素。疏水性的这种差异似乎决定了与膜结合后折叠的肽的分子路径,从而影响其通透性。这项研究有助于更好地理解Sts N端衍生肽的透化活性的分子机制,其含义是在临床环境中利用这些小分子作为全长毒素的替代物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号