当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
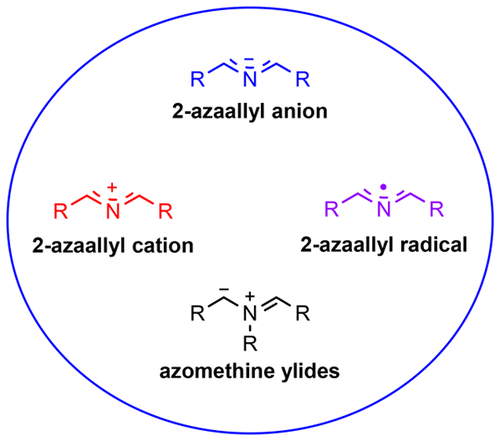
2-Azaallyl Anions, 2-Azaallyl Cations, 2-Azaallyl Radicals, and Azomethine Ylides
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1021/acs.chemrev.8b00349 Shaojian Tang 1 , Xia Zhang 1 , Jiayue Sun 1 , Dawen Niu 1 , Jason J. Chruma 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1021/acs.chemrev.8b00349 Shaojian Tang 1 , Xia Zhang 1 , Jiayue Sun 1 , Dawen Niu 1 , Jason J. Chruma 1
Affiliation
|
This review covers the use of 2-azaallyl anions, 2-azaallyl cations, and 2-azaallyl radicals in organic synthesis up through June 2018. Particular attention is paid to both foundational studies and recent advances over the past decade involving semistabilized and nonstabilized 2-azaallyl anions as key intermediates in various carbon–carbon and carbon–heteroatom bond-forming processes. Both transition-metal-catalyzed and transition-metal-free transformations are covered. Azomethine ylides, which have received significant attention elsewhere, are discussed briefly with the primary focus on critical comparisons with 2-azaallyl anions in regard to generation and use.
中文翻译:

2-氮杂烯丙基阴离子,2-氮杂烯丙基阳离子,2-氮杂烯丙基自由基和氮杂甲叉基内酯
这篇综述涵盖了直至2018年6月在有机合成中使用2-氮杂烯丙基阴离子,2-氮杂烯丙基阳离子和2-氮杂烯丙基自由基。在过去的十年中,基础研究和最近的进展都得到了特别的关注,其中涉及半稳定和不稳定的2-氮杂烯丙基阴离子是各种碳-碳和碳-杂原子键形成过程中的关键中间体。涵盖了过渡金属催化的转化和无过渡金属的转化。简要介绍了在其他地方受到关注的苯甲亚胺基化物,其主要重点是与2-氮杂烯丙基阴离子在生成和使用方面的关键比较。
更新日期:2018-10-10
中文翻译:

2-氮杂烯丙基阴离子,2-氮杂烯丙基阳离子,2-氮杂烯丙基自由基和氮杂甲叉基内酯
这篇综述涵盖了直至2018年6月在有机合成中使用2-氮杂烯丙基阴离子,2-氮杂烯丙基阳离子和2-氮杂烯丙基自由基。在过去的十年中,基础研究和最近的进展都得到了特别的关注,其中涉及半稳定和不稳定的2-氮杂烯丙基阴离子是各种碳-碳和碳-杂原子键形成过程中的关键中间体。涵盖了过渡金属催化的转化和无过渡金属的转化。简要介绍了在其他地方受到关注的苯甲亚胺基化物,其主要重点是与2-氮杂烯丙基阴离子在生成和使用方面的关键比较。











































 京公网安备 11010802027423号
京公网安备 11010802027423号