当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of an Unusual Glycerate Esterification Process in Vioprolide Biosynthesis
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-10-04 00:00:00 , DOI: 10.1021/acschembio.8b00826 David Auerbach 1 , Fu Yan 1 , Youming Zhang 2 , Rolf Müller 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-10-04 00:00:00 , DOI: 10.1021/acschembio.8b00826 David Auerbach 1 , Fu Yan 1 , Youming Zhang 2 , Rolf Müller 1
Affiliation
|
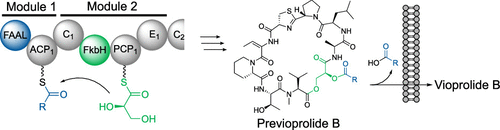
Bacteria produce a large number of secondary metabolites with extraordinary chemical structures and bioactivities. Vioprolides are promising anticancer and antifungal lead compounds produced by the myxobacterium Cystobacter violaceus Cb vi35, which are initially synthesized as acylated precursors (previoprolides) by nonribosomal peptide synthetases (NRPS). Here, we describe and characterize an unprecedented glycerate esterification process in the biosynthesis of vioprolides. In vitro biochemical investigations revealed that the fatty acyl chain of previoprolides is adenylated by the starting fatty acyl-AMP ligase (FAAL) domain, while the glycerate moiety is incorporated by the FkbH domain. An unusual ester-bond forming condensation domain is shown responsible for the acylation of glycerate. LC-MS analysis and bioactivity assays suggest that the acylation serves for directed membrane transport rather than representing a prodrug mechanism.
中文翻译:

Vioprolide生物合成中不寻常的甘油酯化过程的表征
细菌会产生大量具有异常化学结构和生物活性的次级代谢产物。紫罗兰内酯是有希望的抗粘液性紫胶粘杆菌Cb vi35产生的抗癌和抗真菌先导化合物,它们最初是通过非核糖体肽合成酶(NRPS)合成为酰化的前体(前紫罗兰素)。在这里,我们描述和表征了紫罗兰酯的生物合成中前所未有的甘油酸酯化过程。体外生化研究表明,前紫罗兰素的脂肪酰基链被起始脂肪酰基-AMP连接酶(FAAL)域腺苷酸化,而甘油酸酯部分被FkbH域掺入。显示了不寻常的形成酯键的缩合域,该缩合域负责甘油酸酯的酰化。LC-MS分析和生物活性测定表明,酰化作用用于定向膜运输,而不是代表前药机制。
更新日期:2018-10-04
中文翻译:

Vioprolide生物合成中不寻常的甘油酯化过程的表征
细菌会产生大量具有异常化学结构和生物活性的次级代谢产物。紫罗兰内酯是有希望的抗粘液性紫胶粘杆菌Cb vi35产生的抗癌和抗真菌先导化合物,它们最初是通过非核糖体肽合成酶(NRPS)合成为酰化的前体(前紫罗兰素)。在这里,我们描述和表征了紫罗兰酯的生物合成中前所未有的甘油酸酯化过程。体外生化研究表明,前紫罗兰素的脂肪酰基链被起始脂肪酰基-AMP连接酶(FAAL)域腺苷酸化,而甘油酸酯部分被FkbH域掺入。显示了不寻常的形成酯键的缩合域,该缩合域负责甘油酸酯的酰化。LC-MS分析和生物活性测定表明,酰化作用用于定向膜运输,而不是代表前药机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号