当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiol–maleimide “click” chemistry: evaluating the influence of solvent, initiator, and thiol on the reaction mechanism, kinetics, and selectivity†
Polymer Chemistry ( IF 3.9 ) Pub Date : 2015-03-23 00:00:00 , DOI: 10.1039/c5py00168d Brian H. Northrop , Stephen H. Frayne , Umesh Choudhary
Polymer Chemistry ( IF 3.9 ) Pub Date : 2015-03-23 00:00:00 , DOI: 10.1039/c5py00168d Brian H. Northrop , Stephen H. Frayne , Umesh Choudhary
|
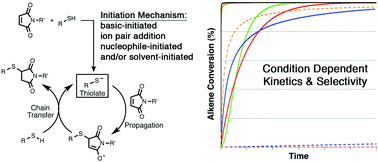
The mechanism and kinetics of thiol–maleimide “click” reactions carried out under a variety of conditions have been investigated computationally and using experimental competition reactions. The influence of three different solvents (chloroform, ethane thiol, and N,N-dimethylformamide), five different initiators (ethylamine, diethylamine, triethylamine, diazabicyclo[2.2.2]octane, and dimethylphenyl-phosphine), and seven different thiols (methyl mercaptan, β-mercaptoethanol, thioacetic acid, methyl thioglycolate, methyl 3-mercaptopropionate, cysteine methyl ester, and thiophenol) on the energetics and kinetics of thiol–maleimide reactions have been examined using density functional methods. Computational and kinetic modeling indicate that the choice of solvent, initiator, and thiol directly influences whether product formation follows a base-, nucleophile-, or ion pair-initiated mechanism (or some combination thereof). The type of mechanism followed determines the overall thiol–maleimide reaction kinetics. Insights from computational studies are then used to understand the selectivity of ternary thiol–maleimide reactions between N-methyl maleimide, thiophenol, and 1-hexanethiol in different combinations of solvents and initiators. The results provide considerable insight into the interplay between reaction conditions, kinetics, and selectivity in thiol–maleimide reactions in particular and thiol-Michael reactions in general, with implications ranging from small molecule synthesis to bioconjugation chemistry and multifunctional materials.
中文翻译:

巯基-马来酰亚胺的“喀哒”化学反应:评估溶剂,引发剂和硫醇对反应机理,动力学和选择性的影响†
在各种条件下进行的巯基-马来酰亚胺“喀哒”反应的机理和动力学已经通过计算和实验竞争反应进行了研究。三种不同溶剂(氯仿,乙烷硫醇和N,N的影响-二甲基甲酰胺),五种不同的引发剂(乙胺,二乙胺,三乙胺,二氮杂双环[2.2.2]辛烷和二甲基苯基膦)和七种不同的硫醇(甲硫醇,β-巯基乙醇,硫代乙酸,巯基乙酸甲酯,3-巯基丙酸甲酯) ,半胱氨酸甲酯和硫酚)对硫醇-马来酰亚胺反应的能量和动力学进行了研究,采用了密度泛函方法。计算和动力学模型表明,溶剂,引发剂和硫醇的选择直接影响产物的形成是否遵循碱,亲核试剂或离子对引发的机理(或其某种组合)。遵循的机理类型决定了总体硫醇-马来酰亚胺反应动力学。在溶剂和引发剂的不同组合中使用N-甲基马来酰亚胺,苯硫酚和1-己硫醇。这些结果提供了对反应条件,动力学和硫醇-马来酰亚胺反应(尤其是硫醇-迈克尔反应)之间选择性的相互作用的广泛见解,涉及的范围从小分子合成到生物共轭化学和多功能材料。
更新日期:2015-03-23
中文翻译:

巯基-马来酰亚胺的“喀哒”化学反应:评估溶剂,引发剂和硫醇对反应机理,动力学和选择性的影响†
在各种条件下进行的巯基-马来酰亚胺“喀哒”反应的机理和动力学已经通过计算和实验竞争反应进行了研究。三种不同溶剂(氯仿,乙烷硫醇和N,N的影响-二甲基甲酰胺),五种不同的引发剂(乙胺,二乙胺,三乙胺,二氮杂双环[2.2.2]辛烷和二甲基苯基膦)和七种不同的硫醇(甲硫醇,β-巯基乙醇,硫代乙酸,巯基乙酸甲酯,3-巯基丙酸甲酯) ,半胱氨酸甲酯和硫酚)对硫醇-马来酰亚胺反应的能量和动力学进行了研究,采用了密度泛函方法。计算和动力学模型表明,溶剂,引发剂和硫醇的选择直接影响产物的形成是否遵循碱,亲核试剂或离子对引发的机理(或其某种组合)。遵循的机理类型决定了总体硫醇-马来酰亚胺反应动力学。在溶剂和引发剂的不同组合中使用N-甲基马来酰亚胺,苯硫酚和1-己硫醇。这些结果提供了对反应条件,动力学和硫醇-马来酰亚胺反应(尤其是硫醇-迈克尔反应)之间选择性的相互作用的广泛见解,涉及的范围从小分子合成到生物共轭化学和多功能材料。























































 京公网安备 11010802027423号
京公网安备 11010802027423号