European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2018-09-27 , DOI: 10.1016/j.ejmech.2018.09.065 Yongshi Yu , Qi Tang , Zhichao Xu , Siliang Li , Mengyu Jin , Zixuan Zhao , Chune Dong , Shuwen Wu , Hai-Bing Zhou
|
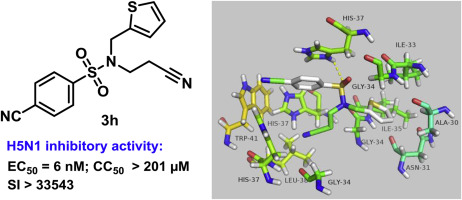
H5N1 virus, one subtype of highly pathogenic influenza A virus in human infection, has recently received attention due to its unpredictable and high mortality. In this study, a series of arylsulfonamide derivatives were identified as improved H5N1 inhibitors for the influenza treatment by systematic structure-activity relationship investigation. Among them, the most potent H5N1 inhibitor 3h exhibited excellent antiviral activity against H5N1 virus with EC50 value of 0.006 μM and selectivity index 33543.3. Moreover, the molecular docking of 3h with M2 proton channel protein provides practical way for understanding the inhibition of H5N1 with this kind of compounds.
中文翻译:

新型强力H5N1抑制剂芳基磺酰胺的合成及其构效关系研究
H5N1病毒是人类感染中高致病性甲型流感病毒的一种亚型,最近由于其不可预测的高死亡率而受到关注。在这项研究中,通过系统的结构-活性关系研究,一系列芳基磺酰胺衍生物被确定为用于流感治疗的改良H5N1抑制剂。其中,最有效的H5N1抑制剂3h对H5N1病毒表现出优异的抗病毒活性,EC 50值为0.006μM,选择性指数为33543.3。此外,3h与M2质子通道蛋白的分子对接为理解这类化合物对H5N1的抑制提供了实用的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号