当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vibrational and Molecular Properties of Mg2+ Binding and Ion Selectivity in the Magnesium Channel MgtE
The Journal of Physical Chemistry B ( IF 2.9 ) Pub Date : 2018-10-10 , DOI: 10.1021/acs.jpcb.8b07967 Tetsunari Kimura , Victor A. Lorenz-Fonfria , Shintaro Douki , Hideyoshi Motoki , Ryuichiro Ishitani , Osamu Nureki , Masahiro Higashi , Yuji Furutani
The Journal of Physical Chemistry B ( IF 2.9 ) Pub Date : 2018-10-10 , DOI: 10.1021/acs.jpcb.8b07967 Tetsunari Kimura , Victor A. Lorenz-Fonfria , Shintaro Douki , Hideyoshi Motoki , Ryuichiro Ishitani , Osamu Nureki , Masahiro Higashi , Yuji Furutani
|
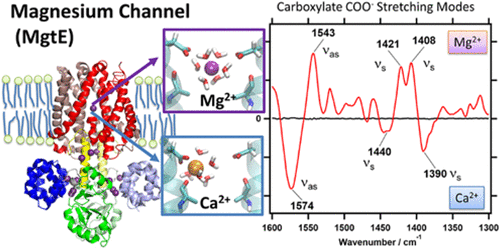
Magnesium ions (Mg2+) are crucial for various biological processes. A bacterial Mg2+ channel, MgtE, tightly regulates the intracellular Mg2+ concentration. Previous X-ray crystal structures showed that MgtE forms a dimeric structure composed of a total of 10 transmembrane α helices forming a central pore, and intracellular soluble domains constituting a Mg2+ sensor. The ion selectivity for Mg2+ over Ca2+ resides at a central cavity in the transmembrane pore of MgtE, involving a conserved aspartate residue (Asp432) from each monomer. Here, we applied ion-exchange-induced difference FTIR spectroscopy to analyze the interactions between MgtE and divalent cations, Mg2+ and Ca2+. Using site-directed mutagenesis, vibrational bands at 1421 (Mg2+), 1407 (Mg2+), ∼1440 (Ca2+), and 1390 (Ca2+) cm–1 were assigned to symmetric carboxylate stretching modes of Asp432, involved in the ion coordination. Conservative modifications of the central cavity by Asp432Glu or Ala417Leu mutations resulted in the disappearance of the Mg2+-sensitive carboxylate bands, suggesting a highly optimized geometry for accommodating a Mg2+ ion. The dependency of the vibrational changes on Mg2+ and Ca2+ concentrations revealed the presence of a two different classes of binding sites: a high affinity site for Mg2+ (Kd ≈ 0.3 mM) with low Ca2+ affinity (Kd ≈ 80 mM), and a medium affinity site for Mg2+ (Kd ≈ 2 mM) and Ca2+ (Kd ≈ 6 mM), tentatively assigned to the central cavity and the sensor domain, respectively. With the aid of molecular dynamics simulation and normal-mode analysis by quantum chemistry, we confirm that changes in carboxylate bands of the high affinity binding site originate from Asp432 in the central cavity.
中文翻译:

镁通道MgtE中Mg 2+结合的振动和分子性质以及离子选择性
镁离子(Mg 2+)对于各种生物过程至关重要。细菌Mg 2+通道MgtE紧密调节细胞内Mg 2+的浓度。先前的X射线晶体结构表明MgtE形成了一个二聚体结构,该结构由总共10个跨膜α螺旋(形成一个中心孔)和构成Mg 2+传感器的细胞内可溶性域组成。Mg 2+对Ca 2+的离子选择性位于MgtE跨膜孔的中心腔中,涉及每个单体的保守的天冬氨酸残基(Asp432)。在这里,我们应用了离子交换诱导的差FTIR光谱分析了MgtE和二价阳离子Mg 2+之间的相互作用。和Ca 2+。使用定点诱变,将1421(Mg 2 +),1407(Mg 2 +),〜1440(Ca 2+)和1390(Ca 2+)cm –1的振动带分配给Asp432的对称羧酸盐拉伸模式,参与离子配位。通过Asp432Glu或Ala417Leu突变对中心腔进行保守修饰,导致Mg 2+敏感的羧酸盐带消失,表明用于容纳Mg 2+离子的高度优化的几何结构。振动变化对Mg 2+和Ca 2+的依赖性浓度揭示了两种不同类型的结合位点的存在:一个高亲和力位点的Mg 2+(ķ d ≈0.3毫摩尔)与低的Ca 2+亲和力(ķ d ≈80毫摩尔),和介质的亲和性的网站的Mg 2 +(ķ d ≈2毫摩尔)和Ca 2+(ķ d ≈6mm)的,暂时分配给所述中央腔和传感器结构域,分别。借助于分子动力学模拟和量子化学的正态分析,我们确认了高亲和力结合位点的羧酸盐带的变化源自中央腔中的Asp432。
更新日期:2018-10-11
中文翻译:

镁通道MgtE中Mg 2+结合的振动和分子性质以及离子选择性
镁离子(Mg 2+)对于各种生物过程至关重要。细菌Mg 2+通道MgtE紧密调节细胞内Mg 2+的浓度。先前的X射线晶体结构表明MgtE形成了一个二聚体结构,该结构由总共10个跨膜α螺旋(形成一个中心孔)和构成Mg 2+传感器的细胞内可溶性域组成。Mg 2+对Ca 2+的离子选择性位于MgtE跨膜孔的中心腔中,涉及每个单体的保守的天冬氨酸残基(Asp432)。在这里,我们应用了离子交换诱导的差FTIR光谱分析了MgtE和二价阳离子Mg 2+之间的相互作用。和Ca 2+。使用定点诱变,将1421(Mg 2 +),1407(Mg 2 +),〜1440(Ca 2+)和1390(Ca 2+)cm –1的振动带分配给Asp432的对称羧酸盐拉伸模式,参与离子配位。通过Asp432Glu或Ala417Leu突变对中心腔进行保守修饰,导致Mg 2+敏感的羧酸盐带消失,表明用于容纳Mg 2+离子的高度优化的几何结构。振动变化对Mg 2+和Ca 2+的依赖性浓度揭示了两种不同类型的结合位点的存在:一个高亲和力位点的Mg 2+(ķ d ≈0.3毫摩尔)与低的Ca 2+亲和力(ķ d ≈80毫摩尔),和介质的亲和性的网站的Mg 2 +(ķ d ≈2毫摩尔)和Ca 2+(ķ d ≈6mm)的,暂时分配给所述中央腔和传感器结构域,分别。借助于分子动力学模拟和量子化学的正态分析,我们确认了高亲和力结合位点的羧酸盐带的变化源自中央腔中的Asp432。























































 京公网安备 11010802027423号
京公网安备 11010802027423号