当前位置:
X-MOL 学术
›
WIREs Comput. Mol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electronic energy transfer in biomacromolecules
Wiley Interdisciplinary Reviews: Computational Molecular Science ( IF 11.4 ) Pub Date : 2018-09-13 , DOI: 10.1002/wcms.1392 Lorenzo Cupellini 1 , Marina Corbella 2 , Benedetta Mennucci 1 , Carles Curutchet 2
Wiley Interdisciplinary Reviews: Computational Molecular Science ( IF 11.4 ) Pub Date : 2018-09-13 , DOI: 10.1002/wcms.1392 Lorenzo Cupellini 1 , Marina Corbella 2 , Benedetta Mennucci 1 , Carles Curutchet 2
Affiliation
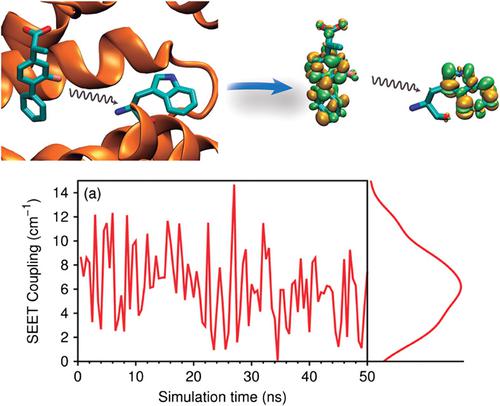
|
Electronic energy transfer is widely used as a molecular ruler to interrogate the structure of biomacromolecules, and performs a key task in photosynthesis by transferring collected energy through specialized pigment–protein complexes. Förster theory, introduced over 70 years ago, allows linking transfer rates to simple structural and spectroscopic properties of the energy‐transferring molecules. In biosystems, however, significant deviations from Förster behavior often arise due to breakdown of the ideal dipole approximation, dielectric screening effects due to the biological environment, or departure from the weak‐coupling regime. In this review, we provide a concise overview of advances in simulations of energy transfer in biomacromolecules that allow overcoming the main limitations of Förster theory. We first discuss advances in quantum chemical methods to compute electronic couplings, their extension to multiscale formulations to include screening effects, and strategies to treat the interplay between coupling fluctuations and energy transfer dynamics. We then examine the spectral overlap term, and how this quantity can be estimated from simulations of the spectral density of exciton–phonon coupling. Finally, we discuss rate theories that can describe energy transfers in situations where strong coupling leads to delocalized excitions, a common situation found in closely packed multichromophoric systems such as photosynthetic complexes and nucleic acids.
中文翻译:

生物大分子中的电子能量转移
电子能量转移被广泛用作询问生物大分子结构的分子标尺,并通过专门的色素-蛋白质复合物转移收集的能量来完成光合作用的关键任务。早在70年前就提出了Förster理论,该理论允许将传递速率与能量传递分子的简单结构和光谱性质联系起来。然而,在生物系统中,由于理想偶极近似的破坏,由于生物环境引起的介电屏蔽效应或背离弱耦合机制,通常会导致与Förster行为的重大偏离。在这篇综述中,我们简要概述了生物大分子中能量转移的模拟进展,从而克服了Förster理论的主要局限。我们首先讨论计算电子耦合的量子化学方法的进展,将其扩展到包括屏蔽效应在内的多尺度公式化,以及讨论耦合波动和能量转移动力学之间相互作用的策略。然后,我们检查频谱重叠项,以及如何通过对激子-声子耦合的频谱密度进行模拟来估计该数量。最后,我们讨论了速率理论,该理论可以描述在强耦合导致离域转移的情况下的能量转移,这是紧密结合的多发色体系(如光合复合物和核酸)中的常见情况。以及解决耦合波动和能量传递动力学之间相互作用的策略。然后,我们检查频谱重叠项,以及如何通过对激子-声子耦合的频谱密度进行模拟来估计该数量。最后,我们讨论了速率理论,该理论可以描述在强耦合导致离域转移的情况下的能量转移,这是紧密结合的多发色体系(如光合复合物和核酸)中的常见情况。以及解决耦合波动和能量传递动力学之间相互作用的策略。然后,我们检查频谱重叠项,以及如何通过对激子-声子耦合的频谱密度进行模拟来估计该数量。最后,我们讨论了速率理论,该理论可以描述在强耦合导致离域转移的情况下的能量转移,这是紧密结合的多发色体系(如光合复合物和核酸)中的常见情况。
更新日期:2018-09-13
中文翻译:

生物大分子中的电子能量转移
电子能量转移被广泛用作询问生物大分子结构的分子标尺,并通过专门的色素-蛋白质复合物转移收集的能量来完成光合作用的关键任务。早在70年前就提出了Förster理论,该理论允许将传递速率与能量传递分子的简单结构和光谱性质联系起来。然而,在生物系统中,由于理想偶极近似的破坏,由于生物环境引起的介电屏蔽效应或背离弱耦合机制,通常会导致与Förster行为的重大偏离。在这篇综述中,我们简要概述了生物大分子中能量转移的模拟进展,从而克服了Förster理论的主要局限。我们首先讨论计算电子耦合的量子化学方法的进展,将其扩展到包括屏蔽效应在内的多尺度公式化,以及讨论耦合波动和能量转移动力学之间相互作用的策略。然后,我们检查频谱重叠项,以及如何通过对激子-声子耦合的频谱密度进行模拟来估计该数量。最后,我们讨论了速率理论,该理论可以描述在强耦合导致离域转移的情况下的能量转移,这是紧密结合的多发色体系(如光合复合物和核酸)中的常见情况。以及解决耦合波动和能量传递动力学之间相互作用的策略。然后,我们检查频谱重叠项,以及如何通过对激子-声子耦合的频谱密度进行模拟来估计该数量。最后,我们讨论了速率理论,该理论可以描述在强耦合导致离域转移的情况下的能量转移,这是紧密结合的多发色体系(如光合复合物和核酸)中的常见情况。以及解决耦合波动和能量传递动力学之间相互作用的策略。然后,我们检查频谱重叠项,以及如何通过对激子-声子耦合的频谱密度进行模拟来估计该数量。最后,我们讨论了速率理论,该理论可以描述在强耦合导致离域转移的情况下的能量转移,这是紧密结合的多发色体系(如光合复合物和核酸)中的常见情况。



























 京公网安备 11010802027423号
京公网安备 11010802027423号