Nutrition & Diabetes ( IF 4.6 ) Pub Date : 2018-09-10 , DOI: 10.1038/s41387-018-0057-6 Y. E. Lentferink , M. P. van der Aa , E. G. A. H. van Mill , C. A. J. Knibbe , M. M. J. van der Vorst
|
Background/Objectives
Off-label metformin is nowadays frequently used for the treatment of obesity in adolescents. However, studies on long-term metformin treatment in adolescents with obesity are scarce. Therefore, an 18 month open label extension study following an 18 months randomized placebo-controlled trial (RCT) on the efficacy, safety, and tolerability of metformin in adolescents with obesity and insulin resistance was performed.
Subjects/Methods
After completion of the RCT, metformin was offered to all participants with a body mass index standard deviation score (BMI-sds) > 2.3 and Homeostasis Model Assessment for Insulin Resistance (HOMA-IR) ≥ 3.4. Endpoints were change in BMI and HOMA-IR.
Results
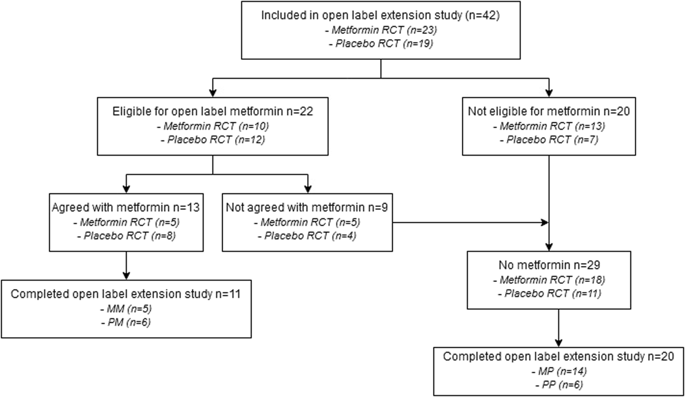
Overall, 31/42 participants completed the extension study (74% girls, median age 14.8 (11.6 – 17.9), BMI 31.2 (22.3 – 45.1), HOMA-IR 3.4 (0.2 – 8.8)). At start, 22/42 (52.4%) participants were eligible for metformin of which 13 (59.0%) agreed with treatment. In participants who continued metformin, an increase was observed in BMI (+2.2 (+0.2 to +9.0)) and HOMA-IR (+13.7 (+1.6 to +48.3)). In metformin naive participants, BMI stabilized after an initial decrease (+0.5 (−2.1 to +5.1)). For HOMA-IR, a decrease was observed (−1.1 (−4.6 to +1.4)).
Conclusion
While metformin treatment in metformin naive participants seems to result in an initial decrease in BMI and HOMA-IR, there is no evidence for sustained effect after prolonged use in adolescents. Limited compliance and/or insufficient dose may explain the differences in long-term effects between adolescents and adults.
中文翻译:

肥胖和胰岛素抵抗青少年的长期二甲双胍治疗,开放标签扩展研究的结果
背景/目标
如今,标签外的二甲双胍经常用于治疗青少年肥胖症。但是,关于肥胖青少年中长期使用二甲双胍治疗的研究很少。因此,在一项为期18个月的随机安慰剂对照试验(RCT)之后,进行了一项18个月的开放标签延伸研究,该试验针对肥胖和胰岛素抵抗青少年中的二甲双胍的疗效,安全性和耐受性。
学科/方法
RCT完成后,向所有参与者提供二甲双胍,其体重指数标准偏差评分(BMI-sds)> 2.3,并且胰岛素抵抗稳态模型评估(HOMA-IR)≥3.4。终点是BMI和HOMA-IR的变化。
结果
总体而言,有31/42名参与者完成了扩展研究(74%的女孩,中位年龄14.8(11.6-17.9),BMI 31.2(22.3-45.1),HOMA-IR 3.4(0.2-8.8))。开始时,有22/42(52.4%)名参与者符合二甲双胍的治疗资格,其中13名(59.0%)同意接受治疗。在继续服用二甲双胍的受试者中,观察到BMI(+2.2(+0.2至+9.0))和HOMA-IR(+13.7(+1.6至+48.3))升高。在初次服用二甲双胍的受试者中,BMI在最初下降后稳定(+0.5(-2.1至+5.1))。对于HOMA-IR,观察到下降(-1.1(-4.6至+1.4))。
结论
虽然在未接受二甲双胍治疗的患者中服用二甲双胍似乎会导致BMI和HOMA-IR的初始下降,但没有证据表明在青少年中长期使用后能持续产生疗效。依从性有限和/或剂量不足可能解释了青少年和成人之间长期影响的差异。











































 京公网安备 11010802027423号
京公网安备 11010802027423号