当前位置:
X-MOL 学术
›
WIREs Comput. Mol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disassembling solvation free energies into local contributions—Toward a microscopic understanding of solvation processes
Wiley Interdisciplinary Reviews: Computational Molecular Science ( IF 16.8 ) Pub Date : 2018-08-30 , DOI: 10.1002/wcms.1390 Matthias Heyden 1
Wiley Interdisciplinary Reviews: Computational Molecular Science ( IF 16.8 ) Pub Date : 2018-08-30 , DOI: 10.1002/wcms.1390 Matthias Heyden 1
Affiliation
|
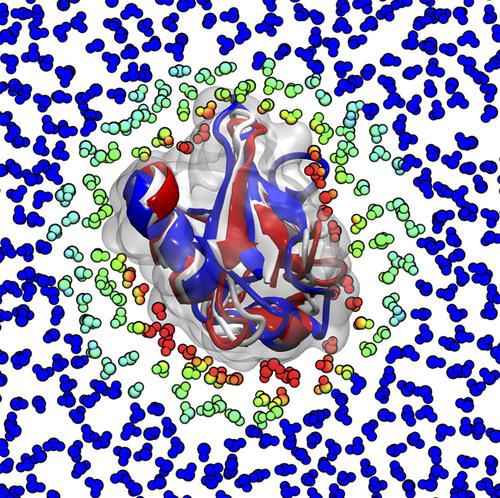
Solvation free energies contribute to the driving force of molecular processes in solution and play a significant role for the relative stability of biomolecular conformations or the formation of complexes. Changes in solvation free energy are the origin of solvent‐mediated interactions such as the hydrophobic effect in water. However, an accurate description of solvation free energies, specifically in aqueous solution, without explicit representation of the solvent is a challenging task in computer simulations. To improve existing models detailed microscopic information on solute–solvent interactions is required, which is not directly accessible from experiments. Explicit solvent simulations include solvent‐mediated effects, however, at a considerable computational cost. Computational tools have been proposed in recent years to extract information on solvation free energies and solute–solvent interactions from explicit solvent simulations. The latter includes spatial decompositions of the solvation enthalpy and entropy, which may eventually lead to an improved theoretical understanding of solvation thermodynamics.
中文翻译:

将溶剂化自由能分解为局部贡献—对溶剂化过程的微观理解
溶剂化自由能有助于溶液中分子过程的驱动力,并且对生物分子构象的相对稳定性或复合物的形成起着重要作用。溶剂化自由能的变化是溶剂介导的相互作用的起源,例如水中的疏水作用。但是,在没有计算机模拟的情况下,准确描述溶剂化自由能(尤其是水溶液中的溶剂化自由能)是一项艰巨的任务。为了改进现有模型,需要有关溶质-溶剂相互作用的详细微观信息,而这些信息不能直接从实验中获得。显式的溶剂模拟包括溶剂介导的效应,但是计算成本很高。近年来,已经提出了计算工具来从明确的溶剂模拟中提取关于溶剂化自由能和溶质-溶剂相互作用的信息。后者包括溶剂化焓和熵的空间分解,最终可能导致对溶剂化热力学的理论理解得到改善。
更新日期:2018-08-30
中文翻译:

将溶剂化自由能分解为局部贡献—对溶剂化过程的微观理解
溶剂化自由能有助于溶液中分子过程的驱动力,并且对生物分子构象的相对稳定性或复合物的形成起着重要作用。溶剂化自由能的变化是溶剂介导的相互作用的起源,例如水中的疏水作用。但是,在没有计算机模拟的情况下,准确描述溶剂化自由能(尤其是水溶液中的溶剂化自由能)是一项艰巨的任务。为了改进现有模型,需要有关溶质-溶剂相互作用的详细微观信息,而这些信息不能直接从实验中获得。显式的溶剂模拟包括溶剂介导的效应,但是计算成本很高。近年来,已经提出了计算工具来从明确的溶剂模拟中提取关于溶剂化自由能和溶质-溶剂相互作用的信息。后者包括溶剂化焓和熵的空间分解,最终可能导致对溶剂化热力学的理论理解得到改善。











































 京公网安备 11010802027423号
京公网安备 11010802027423号