Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lipid Extraction by α-Synuclein Generates Semi-Transmembrane Defects and Lipoprotein Nanoparticles.
ACS Omega ( IF 4.3 ) Pub Date : 2018-08-21 , DOI: 10.1021/acsomega.8b01462 Jianjun Pan , Annalisa Dalzini , Nawal K. Khadka , Chinta M. Aryal , Likai Song
ACS Omega ( IF 4.3 ) Pub Date : 2018-08-21 , DOI: 10.1021/acsomega.8b01462 Jianjun Pan , Annalisa Dalzini , Nawal K. Khadka , Chinta M. Aryal , Likai Song
|
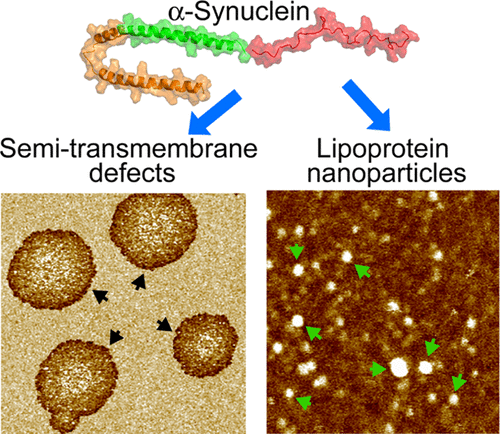
Modulations of synaptic membranes play an essential role in the physiological and pathological functions of the presynaptic protein α-synuclein (αSyn). Here we used solution atomic force microscopy (AFM) and electron paramagnetic resonance (EPR) spectroscopy to investigate membrane modulations caused by αSyn. We used several lipid bilayers to explore how different lipid species may regulate αSyn-membrane interactions. We found that at a protein-to-lipid ratio of ∼1/9, αSyn perturbed lipid bilayers by generating semi-transmembrane defects that only span one leaflet. In addition, αSyn coaggregates with lipid molecules to produce ∼10 nm-sized lipoprotein nanoparticles. The obtained AFM data are consistent with the apolipoprotein characteristic of αSyn. The role of anionic lipids was elucidated by comparing results from zwitterionic and anionic lipid bilayers. Specifically, our AFM measurements showed that anionic bilayers had a larger tendency of forming bilayer defects; similarly, our EPR measurements revealed that anionic bilayers exhibited more substantial changes in lipid chain mobility and bilayer polarity. We also studied the effect of cholesterol. We found that cholesterol increased the capability of αSyn in inducing bilayer defects and altering lipid chain mobility and bilayer polarity. These data can be explained by an increase in the lipid headgroup-headgroup spacing and/or specific cholesterol-αSyn interactions. Interestingly, we found an inhibitory effect of the cone-shaped phosphatidylethanolamine lipids on αSyn-induced bilayer remodeling. We explained our data by considering interlipid hydrogen-bonding that can stabilize bilayer organization and suppress lipid extraction. Our results of lipid-dependent membrane modulations are likely relevant to αSyn functioning.
中文翻译:

α-突触核蛋白的脂质提取产生半跨膜缺陷和脂蛋白纳米颗粒。
突触膜的调节在突触前蛋白α-突触核蛋白(αSyn)的生理和病理功能中起着至关重要的作用。在这里,我们使用溶液原子力显微镜(AFM)和电子顺磁共振(EPR)光谱研究了由αSyn引起的膜调制。我们使用了多个脂质双层来探索不同的脂质种类如何调节αSyn-膜相互作用。我们发现,在蛋白质/脂质比约为1/9的情况下,αSyn通过产生仅跨过一张小叶的半跨膜缺陷来扰动脂质双层。此外,αSyn与脂质分子聚集在一起,产生约10 nm大小的脂蛋白纳米颗粒。所获得的AFM数据与αSyn的载脂蛋白特性一致。通过比较两性离子和阴离子脂质双层的结果,阐明了阴离子脂质的作用。具体而言,我们的原子力显微镜测量结果表明,阴离子双层具有形成双层缺陷的更大趋势。同样,我们的EPR测量结果表明,阴离子双层在脂质链迁移率和双层极性上显示出更多的实质性变化。我们还研究了胆固醇的作用。我们发现胆固醇增加了αSyn诱导双层缺陷并改变脂质链迁移率和双层极性的能力。这些数据可以通过增加脂类首基-首基间隔和/或特定的胆固醇-αSyn相互作用来解释。有趣的是,我们发现了锥形磷脂酰乙醇胺脂质对αSyn诱导的双层重塑的抑制作用。我们通过考虑可以稳定双层组织并抑制脂质提取的脂间氢键解释了我们的数据。我们的脂质依赖性膜调节结果可能与αSyn功能有关。
更新日期:2018-08-21
中文翻译:

α-突触核蛋白的脂质提取产生半跨膜缺陷和脂蛋白纳米颗粒。
突触膜的调节在突触前蛋白α-突触核蛋白(αSyn)的生理和病理功能中起着至关重要的作用。在这里,我们使用溶液原子力显微镜(AFM)和电子顺磁共振(EPR)光谱研究了由αSyn引起的膜调制。我们使用了多个脂质双层来探索不同的脂质种类如何调节αSyn-膜相互作用。我们发现,在蛋白质/脂质比约为1/9的情况下,αSyn通过产生仅跨过一张小叶的半跨膜缺陷来扰动脂质双层。此外,αSyn与脂质分子聚集在一起,产生约10 nm大小的脂蛋白纳米颗粒。所获得的AFM数据与αSyn的载脂蛋白特性一致。通过比较两性离子和阴离子脂质双层的结果,阐明了阴离子脂质的作用。具体而言,我们的原子力显微镜测量结果表明,阴离子双层具有形成双层缺陷的更大趋势。同样,我们的EPR测量结果表明,阴离子双层在脂质链迁移率和双层极性上显示出更多的实质性变化。我们还研究了胆固醇的作用。我们发现胆固醇增加了αSyn诱导双层缺陷并改变脂质链迁移率和双层极性的能力。这些数据可以通过增加脂类首基-首基间隔和/或特定的胆固醇-αSyn相互作用来解释。有趣的是,我们发现了锥形磷脂酰乙醇胺脂质对αSyn诱导的双层重塑的抑制作用。我们通过考虑可以稳定双层组织并抑制脂质提取的脂间氢键解释了我们的数据。我们的脂质依赖性膜调节结果可能与αSyn功能有关。























































 京公网安备 11010802027423号
京公网安备 11010802027423号