Biochimie ( IF 3.3 ) Pub Date : 2018-08-06 , DOI: 10.1016/j.biochi.2018.07.022 Carolline Fernanda Rodrigues Ascenção , Raghavendra Sashi Krishna Nagampalli , Zeyaul Islam , Matheus Pinto Pinheiro , Larissa Menezes dos Reis , Bianca Alves Pauletti , Carolina Aparecida de Guzzi Cassago , Daniela Campos Granato , Adriana Franco Paes Leme , Sandra Martha Gomes Dias
|
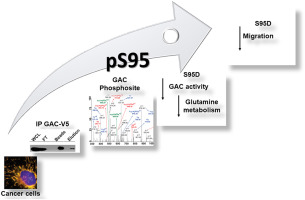
The mitochondrial phosphate-activated glutaminase C (GAC) is produced by the alternative splicing of the GLS gene. Compared to the other GLS isoform, the kidney-type glutaminase (KGA), GAC is more enzymatically efficient and of particular importance for cancer cell growth. Although its catalytic mechanism is well understood, little is known about how post-translational modifications can impact GAC function. Here, we identified by mass spectrometry a phosphorylated serine at the GLS N-terminal domain (at position 95) and investigated its role on regulating GAC activity. The ectopic expression of the phosphomimetic mutant (GAC.S95D) in breast cancer cells, compared to wild-type GAC (GAC.WT), led to decreased glutaminase activity, glutamine uptake, glutamate release and intracellular glutamate levels, without changing GAC sub-cellular localization. Interestingly, cells expressing the GAC.S95D mutant, compared to GAC.WT, presented decreased migration and vimentin level, an epithelial-to-mesenchymal transition marker. These results reveal that GAC is post-translationally regulated by phosphorylation, which affects cellular glutamine metabolism and glutaminase-related cell phenotype.
中文翻译:

谷氨酰胺酶C的N末端磷酸化降低其酶活性和癌细胞迁移
线粒体磷酸激活的谷氨酰胺酶C(GAC)是通过GLS基因的选择性剪接产生的。与其他GLS相比异构体,即肾型谷氨酰胺酶(KGA),GAC在酶促方面更为有效,对于癌细胞的生长尤为重要。尽管其催化机理已广为人知,但对翻译后修饰如何影响GAC功能的了解甚少。在这里,我们通过质谱法鉴定了位于GLS N末端结构域(位于95位)的磷酸化丝氨酸,并研究了其在调节GAC活性中的作用。与野生型GAC(GAC.WT)相比,乳腺癌细胞中模拟磷酸化突变体(GAC.S95D)的异位表达导致谷氨酰胺酶活性,谷氨酰胺吸收,谷氨酸释放和细胞内谷氨酸水平降低,而不会改变GAC亚细胞定位。有趣的是,与GAC.WT相比,表达GAC.S95D突变体的细胞呈现出降低的迁移和波形蛋白水平,上皮到间质的过渡标记。这些结果表明,GAC在翻译后受磷酸化调节,从而影响细胞的谷氨酰胺代谢和谷氨酰胺酶相关的细胞表型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号