当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bacteria as an Electron Shuttle for Sulfide Oxidation.
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2018-07-31 , DOI: 10.1021/acs.estlett.8b00319 Annemiek Ter Heijne 1 , Rieks de Rink 1, 2 , Dandan Liu 1 , Johannes B M Klok 1, 2, 3 , Cees J N Buisman 1, 3
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2018-07-31 , DOI: 10.1021/acs.estlett.8b00319 Annemiek Ter Heijne 1 , Rieks de Rink 1, 2 , Dandan Liu 1 , Johannes B M Klok 1, 2, 3 , Cees J N Buisman 1, 3
Affiliation
|
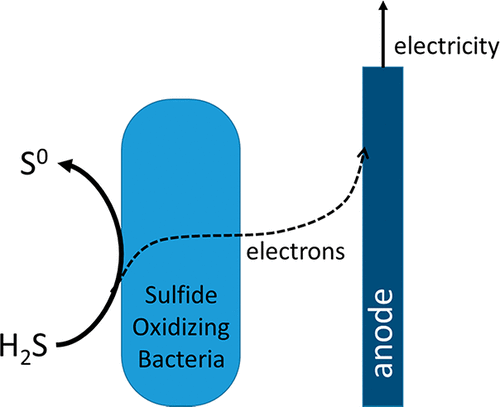
Biological desulfurization under haloalkaliphilic conditions is a widely applied process, in which haloalkalophilic sulfide-oxidizing bacteria (SOB) oxidize dissolved sulfide with oxygen as the final electron acceptor. We show that these SOB can shuttle electrons from sulfide to an electrode, producing electricity. Reactor solutions from two different biodesulfurization installations were used, containing different SOB communities; 0.2 mM sulfide was added to the reactor solutions with SOB in absence of oxygen, and sulfide was removed from the solution. Subsequently, the reactor solutions with SOB, and the centrifuged reactor solutions without SOB, were transferred to an electrochemical cell, where they were contacted with an anode. Charge recovery was studied at different anode potentials. At an anode potential of +0.1 V versus Ag/AgCl, average current densities of 0.48 and 0.24 A/m2 were measured for the two reactor solutions with SOB. Current was negligible for reactor solutions without SOB. We postulate that these differences in current are related to differences in microbial community composition. Potential mechanisms for charge storage in SOB are proposed. The ability of SOB to shuttle electrons from sulfide to an electrode offers new opportunities for developing a more sustainable desulfurization process.
中文翻译:

细菌作为用于硫化物氧化的电子梭。
卤化嗜碱条件下的生物脱硫是一个广泛应用的过程,其中卤化嗜碱性硫化物氧化细菌(SOB)用氧气作为最终电子受体氧化溶解的硫化物。我们证明了这些SOB可以将电子从硫化物传递到电极上,从而产生电。使用了来自两个不同生物脱硫装置的反应器解决方案,其中包含不同的SOB群落。在无氧的情况下,使用SOB将0.2 mM硫化物添加到反应器溶液中,并从溶液中除去硫化物。随后,将具有SOB的反应器溶液和不具有SOB的离心反应器溶液转移到电化学电池中,在此它们与阳极接触。在不同的阳极电位下研究了电荷恢复。相对于Ag / AgCl,阳极电势为+0.1 V时,使用SOB的两种反应器溶液的平均电流密度分别为0.48和0.24 A / m2。对于没有SOB的反应器解决方案,电流可以忽略不计。我们假设电流的这些差异与微生物群落组成的差异有关。提出了SOB中电荷存储的潜在机制。SOB将电子从硫化物传递到电极的能力为开发更具可持续性的脱硫工艺提供了新的机会。
更新日期:2018-08-03
中文翻译:

细菌作为用于硫化物氧化的电子梭。
卤化嗜碱条件下的生物脱硫是一个广泛应用的过程,其中卤化嗜碱性硫化物氧化细菌(SOB)用氧气作为最终电子受体氧化溶解的硫化物。我们证明了这些SOB可以将电子从硫化物传递到电极上,从而产生电。使用了来自两个不同生物脱硫装置的反应器解决方案,其中包含不同的SOB群落。在无氧的情况下,使用SOB将0.2 mM硫化物添加到反应器溶液中,并从溶液中除去硫化物。随后,将具有SOB的反应器溶液和不具有SOB的离心反应器溶液转移到电化学电池中,在此它们与阳极接触。在不同的阳极电位下研究了电荷恢复。相对于Ag / AgCl,阳极电势为+0.1 V时,使用SOB的两种反应器溶液的平均电流密度分别为0.48和0.24 A / m2。对于没有SOB的反应器解决方案,电流可以忽略不计。我们假设电流的这些差异与微生物群落组成的差异有关。提出了SOB中电荷存储的潜在机制。SOB将电子从硫化物传递到电极的能力为开发更具可持续性的脱硫工艺提供了新的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号