Biochimie ( IF 3.3 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.biochi.2018.07.024 Nitish Jangde , Rashmi Ray , Sunita Sinha , Khokan Rana , Satyendra Kumar Singh , Prashant Khandagale , Narottam Acharya , Vivek Rai
|
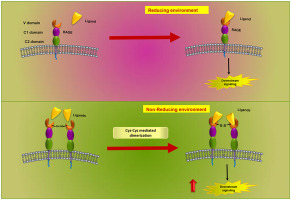
Receptor for Advanced Glycation End product (RAGE) is a multiligand receptor implicated in diverse pathological conditions such as diabetes, atherosclerosis, cancer and neural diseases. Extracellular, RAGE consists of V, C1 and C2 domains. Here, we show RAGE exists as a monomer in equilibrium with a fraction of a covalently linked dimer of monomers via its V domain through cysteine. In order to understand the functional implication of this dimer, we examined the binding capacity and functional potential of RAGE dimer via advanced glycation end products (AGEs) which shows enhanced binding capacity towards V domain, ERK phosphorylation, cytokine release and actin polymerization ability of the dimeric form for AGEs compared with the reduced monomeric form. Our data, suggests that the dimeric state of RAGE controls its function and ligand mediated signaling which may play important role in RAGE mediated various diseases.
中文翻译:

半胱氨酸介导的RAGE V结构域中的二硫键形成促进其功能相关的二聚化
晚期糖基化终产物(RAGE)的受体是一种多配体受体,涉及多种病理状况,例如糖尿病,动脉粥样硬化,癌症和神经疾病。在细胞外,RAGE由V,C1和C2域组成。在这里,我们显示RAGE作为单体存在,与一部分通过半胱氨酸的V结构域共价连接的单体二聚体处于平衡状态。为了了解该二聚体的功能含义,我们通过高级糖基化终产物(AGEs)检验了RAGE二聚体的结合能力和功能潜能,该产物显示了其对V结构域,ERK磷酸化,细胞因子释放和肌动蛋白聚合能力的增强结合能力。与还原的单体形式相比,AGEs的二聚体形式。我们的数据











































 京公网安备 11010802027423号
京公网安备 11010802027423号