当前位置:
X-MOL 学术
›
Mol. Omics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural motif, topi and its role in protein function and fibrillation
Molecular Omics ( IF 3.0 ) Pub Date : 2018-06-05 , DOI: 10.1039/c8mo00048d Jesmita Dhar 1, 2, 3, 4 , Pinak Chakrabarti 1, 2, 3, 4, 5
Molecular Omics ( IF 3.0 ) Pub Date : 2018-06-05 , DOI: 10.1039/c8mo00048d Jesmita Dhar 1, 2, 3, 4 , Pinak Chakrabarti 1, 2, 3, 4, 5
Affiliation
|
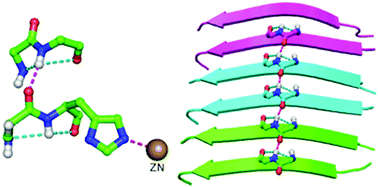
A protein chain is arranged into regions in which the backbone is organized into regular patterns (of conformation and hydrogen bonding) to form the most common secondary structures, α-helix and β-sheet, which are interspersed by turns and more irregular loop regions. A structural motif, topi, is discussed in which a pair of 2-residue segments, each containing hydrogen-bonded five-membered fused-ring motifs, distant in sequence are linked to each other by a hydrogen bond. Though a small motif, it appears to be important in the context of local folding patterns of proteins and occurs near protein active sites. The motif shows quite significant residue preference, and a Cys (or Ser) occupying the second position may further stabilize the motif by forming an additional hydrogen bond across it. Remarkably, topi is found within disease causing misfolded proteins, such as the fibrilled form of Aβ42, and also across the interface between two protein chains. This motif may be an important component of fibrillation and useful for modeling loop regions.
中文翻译:

结构基序,托皮及其在蛋白质功能和原纤维形成中的作用
蛋白质链排列在区域中,在该区域中,主链被组织成规则的图案(构象和氢键),以形成最常见的二级结构,α-螺旋和β-折叠,其间散布着转弯和更不规则的环区。讨论了一个结构基序topi,其中一对2个残基片段(每个都包含顺序隔开的氢键合的五元稠合环基序)通过氢键相互连接。尽管是一个很小的基序,但在蛋白质的局部折叠模式中它似乎很重要,并且发生在蛋白质活性位点附近。该基序表现出相当显着的残基偏好,并且占据第二位置的Cys(或Ser)可以通过在基序上形成额外的氢键来进一步稳定基序。值得注意的是托皮是疾病中发现的引起错折叠蛋白,例如Aβ42的fibrilled形式,并且还跨越两条蛋白链之间的接口。该基序可能是原纤化的重要组成部分,可用于建模环区域。
更新日期:2018-12-01
中文翻译:

结构基序,托皮及其在蛋白质功能和原纤维形成中的作用
蛋白质链排列在区域中,在该区域中,主链被组织成规则的图案(构象和氢键),以形成最常见的二级结构,α-螺旋和β-折叠,其间散布着转弯和更不规则的环区。讨论了一个结构基序topi,其中一对2个残基片段(每个都包含顺序隔开的氢键合的五元稠合环基序)通过氢键相互连接。尽管是一个很小的基序,但在蛋白质的局部折叠模式中它似乎很重要,并且发生在蛋白质活性位点附近。该基序表现出相当显着的残基偏好,并且占据第二位置的Cys(或Ser)可以通过在基序上形成额外的氢键来进一步稳定基序。值得注意的是托皮是疾病中发现的引起错折叠蛋白,例如Aβ42的fibrilled形式,并且还跨越两条蛋白链之间的接口。该基序可能是原纤化的重要组成部分,可用于建模环区域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号