npj Precision Oncology ( IF 6.8 ) Pub Date : 2018-05-08 , DOI: 10.1038/s41698-018-0056-z Rhian M Touyz 1 , Joerg Herrmann 2
|
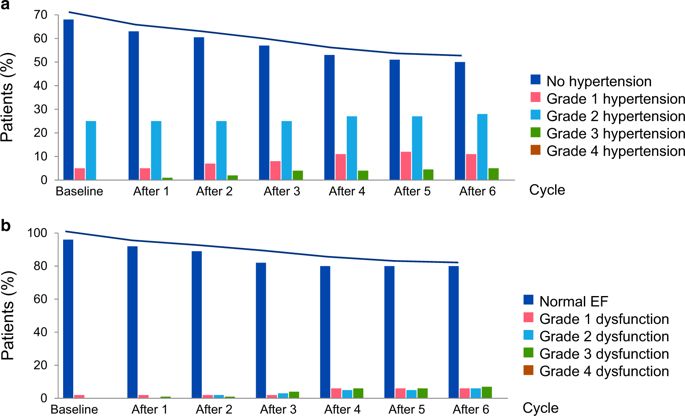
Angiogenesis inhibitors targeting the vascular endothelial growth factor (VEGF) signaling pathway (VSP) have been important additions in the therapy of various cancers, especially renal cell carcinoma and colorectal cancer. Bevazicumab, the first VSP to receive FDA approval in 2004 targeting all circulating isoforms of VEGF-A, has become one of the best-selling drugs of all times. The second wave of tyrosine kinase inhibitors (TKIs), which target the intracellular site of VEGF receptor kinases, began with the approval of sorafenib in 2005 and sunitinib in 2006. Heart failure was subsequently noted, in 2–4% of patients on bevacizumab and in 3–8% of patients on VSP-TKIs. The very fact that the single-targeted monoclonal antibody bevacizumab can induce cardiotoxicity supports a pathomechanistic role for the VSP and the postulate of the “vascular” nature of VSP inhibitor cardiotoxicity. In this review we will outline this scenario in greater detail, reflecting on hypertension and coronary artery disease as risk factors for VSP inhibitor cardiotoxicity, but also similarities with peripartum and diabetic cardiomyopathy. This leads to the concept that any preexisting or coexisting condition that reduces the vascular reserve or utilizes the vascular reserve for compensatory purposes may pose a risk factor for cardiotoxicity with VSP inhibitors. These conditions need to be carefully considered in cancer patients who are to undergo VSP inhibitor therapy. Such vigilance is not to exclude patients from such prognostically extremely important therapy but to understand the continuum and to recognize and react to any cardiotoxicity dynamics early on for superior overall outcomes.
中文翻译:

血管内皮生长因子抑制剂治疗的心脏毒性
针对血管内皮生长因子(VEGF)信号通路(VSP)的血管生成抑制剂已成为各种癌症(尤其是肾细胞癌和结直肠癌)治疗中的重要补充。 Bevazicumab 是第一个于 2004 年获得 FDA 批准的针对所有循环 VEGF-A 亚型的 VSP,现已成为有史以来最畅销的药物之一。第二波酪氨酸激酶抑制剂(TKI)以 VEGF 受体激酶的细胞内位点为靶点,始于 2005 年索拉非尼和 2006 年舒尼替尼的批准。随后注意到,使用贝伐珠单抗和舒尼替尼的患者中有 2-4% 出现心力衰竭。 3-8% 接受 VSP-TKI 治疗的患者出现这种情况。单靶点单克隆抗体贝伐单抗可诱导心脏毒性这一事实支持了 VSP 的病理机制作用以及 VSP 抑制剂心脏毒性的“血管”性质的假设。在这篇综述中,我们将更详细地概述这种情况,反映高血压和冠状动脉疾病作为 VSP 抑制剂心脏毒性的危险因素,但也与围产期和糖尿病心肌病相似。这导致了这样的概念:任何减少血管储备或利用血管储备进行代偿的先前存在或共存的病症都可能构成 VSP 抑制剂心脏毒性的危险因素。对于接受 VSP 抑制剂治疗的癌症患者,需要仔细考虑这些情况。这种警惕并不是为了将患者排除在这种对预后极其重要的治疗之外,而是为了了解连续性并尽早识别任何心脏毒性动态并对其做出反应,以获得更好的总体结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号