npj Parkinson's Disease ( IF 6.7 ) Pub Date : 2018-04-19 , DOI: 10.1038/s41531-018-0049-1 Jillian H. Kluss , Melissa M. Conti , Alice Kaganovich , Aleksandra Beilina , Heather L. Melrose , Mark R. Cookson , Adamantios Mamais
|
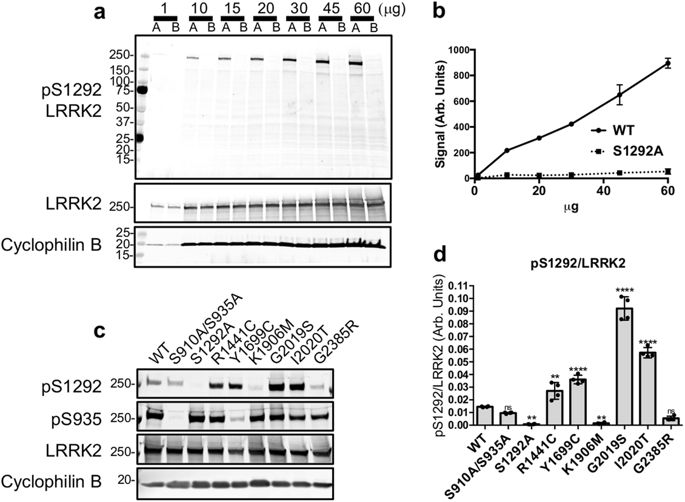
Parkinson’s disease-linked mutations in LRRK2 enhance the kinase activity of the protein, therefore targeting LRRK2 kinase activity is a promising therapeutic approach. Phosphorylation at S935 of LRRK2 and of its Rab GTPase substrates have proven very useful biomarkers to monitor its kinase activity. Complementary to these approaches autophosphorylation of LRRK2 can be used as a direct kinase activity readout but to date detection of autophosphorylation at endogenous levels in vivo has been limited. We developed a fractionation-based enrichment method to successfully detect endogenous S1292 LRRK2 autophosphorylation in mouse tissues and highlight S1292 as a physiological readout candidate for LRRK2 kinase activity in vivo.
中文翻译:

检测小鼠组织中的内源性S1292 LRRK2自磷酸化作为激酶活性的读数
帕金森病在LRRK2中的疾病相关突变增强了该蛋白的激酶活性,因此靶向LRRK2激酶活性是一种有前途的治疗方法。LRRK2及其Rab GTPase底物在S935处的磷酸化已被证明是非常有用的生物标记,可监测其激酶活性。作为这些方法的补充,LRRK2的自磷酸化可以用作直接的激酶活性读数,但是迄今为止,在体内内源水平检测自磷酸化已经受到限制。我们开发了基于分馏的富集方法,以成功检测小鼠组织中的内源性S1292 LRRK2自磷酸化,并强调了S1292作为体内LRRK2激酶活性的生理候选物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号