Redox Biology ( IF 10.7 ) Pub Date : 2020-01-02 , DOI: 10.1016/j.redox.2019.101419 Naijin Zhang 1 , Ying Zhang 1 , Boquan Wu 1 , Shilong You 1 , Yingxian Sun 1
|
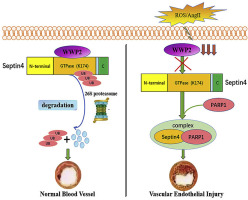
Oxidative stress-associated endothelial injury is the initial event and major cause of multiple cardiovascular diseases such as atherosclerosis and hypertensive angiopathy. A protein homeostasis imbalance is a critical cause of endothelial injury, and homologous to E6AP C-terminus (HECT)-type E3 ubiquitin ligases are the core factors controlling protein homeostasis. Although HECT-type E3 ubiquitin ligases are involved in the regulation of cardiac development and diseases, their roles in endothelial injury remain largely unknown. This study aimed to identify which HECT-type E3 ubiquitin ligase is involved in endothelial injury and clarify the mechanisms at molecular, cellular, and organism levels. We revealed a novel role of the HECT-type E3 ubiquitin ligase WWP2 in regulating endothelial injury and vascular remodeling after endothelial injury. Endothelial/myeloid-specific WWP2 knockout in mice significantly aggravated angiotensin II/oxidative stress-induced endothelial injury and vascular remodeling after endothelial injury. The same results were obtained from in vitro experiments. Mechanistically, the endothelial injury factor Septin4 was identified as a novel physiological substrate of WWP2. In addition, WWP2 interacted with the GTPase domain of Septin4, ubiquitinating Septin4-K174 to degrade Septin4 through the ubiquitin-proteasome system, which inhibited the Septin4-PARP1 endothelial damage complex. These results identified the first endothelial injury-associated physiological pathway regulated by HECT-type E3 ubiquitin ligases in vivo as well as a unique proteolytic mechanism through which WWP2 controls endothelial injury and vascular remodeling after endothelial injury. These findings might provide a novel treatment strategy for oxidative stress-associated atherosclerosis and hypertensive vascular diseases.
中文翻译:

WW域E3泛素蛋白连接酶2在调节氧化应激内皮损伤中Septin4的泛素化和降解中的作用。
氧化应激相关的内皮损伤是多种心血管疾病(如动脉粥样硬化和高血压性血管病)的初始事件和主要原因。蛋白质稳态失衡是内皮损伤的关键原因,与E6AP C端(HECT)型E3泛素连接酶同源是控制蛋白质稳态的核心因素。尽管HECT型E3泛素连接酶参与了心脏发育和疾病的调节,但它们在内皮损伤中的作用仍然未知。这项研究旨在确定哪种HECT型E3泛素连接酶参与了内皮损伤,并阐明了在分子,细胞和生物体水平上的机制。我们揭示了HECT型E3泛素连接酶WWP2在调节内皮损伤和内皮损伤后血管重塑中的新作用。小鼠的内皮/髓样特异性WWP2敲除显着加重了血管紧张素II /氧化应激诱导的内皮损伤和内皮损伤后的血管重塑。从体外实验获得了相同的结果。从机制上讲,内皮损伤因子Septin4被确定为WWP2的新型生理底物。此外,WWP2与Septin4的GTPase结构域相互作用,泛素化Septin4-K174以通过泛素-蛋白酶体系统降解Septin4,从而抑制Septin4-PARP1内皮损伤复合体。这些结果确定了由HECT型E3泛素连接酶调控的第一个内皮损伤相关的生理途径 从体外实验获得了相同的结果。从机制上讲,内皮损伤因子Septin4被确定为WWP2的新型生理底物。此外,WWP2与Septin4的GTPase结构域相互作用,泛素化Septin4-K174以通过泛素-蛋白酶体系统降解Septin4,从而抑制Septin4-PARP1内皮损伤复合物。这些结果确定了由HECT型E3泛素连接酶调控的第一个内皮损伤相关的生理途径 从体外实验获得了相同的结果。从机制上讲,内皮损伤因子Septin4被确定为WWP2的新型生理底物。此外,WWP2与Septin4的GTPase结构域相互作用,泛素化Septin4-K174以通过泛素-蛋白酶体系统降解Septin4,从而抑制Septin4-PARP1内皮损伤复合体。这些结果确定了由HECT型E3泛素连接酶调控的第一个内皮损伤相关的生理途径 抑制Septin4-PARP1内皮损伤复合物。这些结果确定了由HECT型E3泛素连接酶调控的第一个内皮损伤相关的生理途径 抑制Septin4-PARP1内皮损伤复合物。这些结果确定了由HECT型E3泛素连接酶调控的第一个内皮损伤相关的生理途径WWP2控制着内皮损伤和内皮损伤后的血管重塑的独特体内蛋白水解机制。这些发现可能为氧化应激相关的动脉粥样硬化和高血压血管疾病提供一种新颖的治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号