当前位置:
X-MOL 学术
›
ACS Mater. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of I2 on Cu2–xS Nanoparticles: Enabling Cation Exchange but Complicating Plasmonics
ACS Materials Letters ( IF 9.6 ) Pub Date : 2020-01-06 , DOI: 10.1021/acsmaterialslett.9b00402 Han K. D. Le 1 , Huiyan Xiong 1 , Bonnie A. Page 1 , Luis F. Garcia-Herrera 1 , Haley P. McAllister 1 , Boxi Cameron Li 1 , Haiying Wang 2 , Katherine E. Plass 1
ACS Materials Letters ( IF 9.6 ) Pub Date : 2020-01-06 , DOI: 10.1021/acsmaterialslett.9b00402 Han K. D. Le 1 , Huiyan Xiong 1 , Bonnie A. Page 1 , Luis F. Garcia-Herrera 1 , Haley P. McAllister 1 , Boxi Cameron Li 1 , Haiying Wang 2 , Katherine E. Plass 1
Affiliation
|
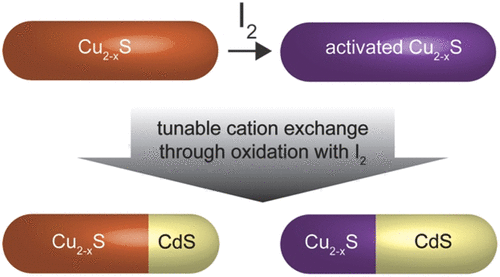
The effects of I2 oxidation on the behavior of Cu2xS nanoparticles—their long-term localized-surface plasmon resonance (LSPR), crystal structure, composition, and ability to undergo cation exchange—were explored. I2 has been known to induce a blue shift in the LSPR of Cu2xS nanoparticles due to the increase in the hole concentration through the removal of Cu+ ions. An unexpected red shift is observed in I2-exposed Cu2–xS nanoparticles over the longer times required for cation exchange. Despite this complication of the LSPR behavior, the process of cation exchange from Cu+ to Cd2+ is modulated by the exposure of the Cu2xS nanoparticle precursor to elemental iodide, I2. This is consistent with the promotion of cation exchange through increased vacancy concentration. Treatment with I2 induces a greater extent of cation exchange than in controls. Increasing I2 exposure results in a greater extent of exchange and exchange at lower temperatures. The increased mobility of sulfide ions to which the red shift in LSPR is attributed also affects the resultant CdS crystal structure. Zinc-blende CdS, which has an altered anion network, is observed upon increased I2 exposure or extended cation-exchange reaction times. This work offers insights into the mechanism of cation exchange and the modulation of the LSPR of plasmonic semiconductors.
中文翻译:

I 2对Cu 2– x S纳米粒子的影响:能够进行阳离子交换,但使等离子体运动复杂化。
研究了I 2氧化对Cu 2 x S纳米粒子行为的影响-纳米粒子的长期局部表面等离子体共振(LSPR),晶体结构,组成和进行阳离子交换的能力。已知I 2会导致Cu 2 x S纳米粒子的LSPR发生蓝移,这是由于通过去除Cu +离子导致空穴浓度增加所致。在暴露于I 2的Cu 2– x S纳米颗粒中,在进行阳离子交换所需的较长时间上观察到了意外的红移。尽管LSPR行为很复杂,但阳离子从Cu +到Cd 2+的交换过程通过将Cu 2 x S纳米颗粒前体暴露于元素碘化物I 2来调节。这与通过增加空位浓度促进阳离子交换相一致。与对照相比,用I 2处理诱导更大程度的阳离子交换。I 2暴露的增加导致更大程度的交换和在较低温度下的交换。归因于LSPR的红移的硫化物离子迁移率的提高,也会影响所得的CdS晶体结构。当I 2增加时,可以观察到具有改变的阴离子网络的闪锌矿CdS。暴露或延长阳离子交换反应时间。这项工作提供了对阳离子交换和等离激元半导体的LSPR的调制机制的见解。
更新日期:2020-01-07
中文翻译:

I 2对Cu 2– x S纳米粒子的影响:能够进行阳离子交换,但使等离子体运动复杂化。
研究了I 2氧化对Cu 2 x S纳米粒子行为的影响-纳米粒子的长期局部表面等离子体共振(LSPR),晶体结构,组成和进行阳离子交换的能力。已知I 2会导致Cu 2 x S纳米粒子的LSPR发生蓝移,这是由于通过去除Cu +离子导致空穴浓度增加所致。在暴露于I 2的Cu 2– x S纳米颗粒中,在进行阳离子交换所需的较长时间上观察到了意外的红移。尽管LSPR行为很复杂,但阳离子从Cu +到Cd 2+的交换过程通过将Cu 2 x S纳米颗粒前体暴露于元素碘化物I 2来调节。这与通过增加空位浓度促进阳离子交换相一致。与对照相比,用I 2处理诱导更大程度的阳离子交换。I 2暴露的增加导致更大程度的交换和在较低温度下的交换。归因于LSPR的红移的硫化物离子迁移率的提高,也会影响所得的CdS晶体结构。当I 2增加时,可以观察到具有改变的阴离子网络的闪锌矿CdS。暴露或延长阳离子交换反应时间。这项工作提供了对阳离子交换和等离激元半导体的LSPR的调制机制的见解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号