Chemical Engineering Research and Design ( IF 3.7 ) Pub Date : 2020-01-02 , DOI: 10.1016/j.cherd.2019.12.025 Xiaoqing Dang , Shijie Li , Xin Yu , Hui Guo , Caihong Qin , Li Cao
|
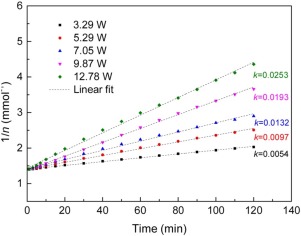
This study was designed to evaluate the effectiveness of adsorbed toluene removal using a coaxial cylindrical dielectric barrier discharge (DBD) reactor packed with a hybrid material catalyst [M/13X-γ-Al2O3 (M: Ag, Ce, Mn, and Co)]. All experiments were conducted at atmosphere pressure and room temperature. Results showed that Ag/13X-γ-Al2O3 (Ag/13X-Al) exhibited the highest breakthrough capacity (˜2.14 mmol) and the highest mineralization rate (MR) for toluene decomposition (˜68%) at 20 kV. The kinetic model on adsorbed toluene mineralization showed a good fit with the pseudo-second-order kinetic model for all catalysts. The model determined by discharge power (P) and initial amount of adsorbed toluene (n0) is expressed as: , where t and n denote the reaction time and the amount of adsorbed toluene, respectively. Finally, the model reliability was verified by comparing the experimental values of MR with the calculated values.
中文翻译:

吸附除去甲苯的动力学鉴定涉及杂化材料catalysts- [M / 13X-γ-Al系2 ö 3(M:银,铈,Mn和Co)]以顺序的非热等离子体的系统
本研究旨在使用同轴圆筒形电介质势垒放电,以评估吸附除去甲苯的有效性(DBD)反应器填充有混合材料催化剂[M / 13X-γ-Al系2 ö 3(M:银,铈,锰,和Co)]。所有实验均在大气压和室温下进行。结果表明,银/ 13X-γ-Al系2 ö 3银(Ag / 13X-AL)在20kV显示出对甲苯分解(~68%)最高的穿透容量(~2.14毫摩尔)和最高矿化速率(MR)。吸附甲苯矿化的动力学模型与所有催化剂的拟二级动力学模型都很好地吻合。该模型由放电功率(P)和甲苯的初始吸附量(n 0)表示为:,其中t和n分别表示反应时间和甲苯吸附量。最后,通过将MR的实验值与计算值进行比较来验证模型的可靠性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号