当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of tandem biotransformation for biosynthesis of new pentacyclic triterpenoid derivatives with neuroprotective effect.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-01-02 , DOI: 10.1016/j.bmcl.2019.126947 Shao-Hua Xu 1 , Hai-Lan Chen 1 , Yong Fan 2 , Wei Xu 1 , Jian Zhang 3
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-01-02 , DOI: 10.1016/j.bmcl.2019.126947 Shao-Hua Xu 1 , Hai-Lan Chen 1 , Yong Fan 2 , Wei Xu 1 , Jian Zhang 3
Affiliation
|
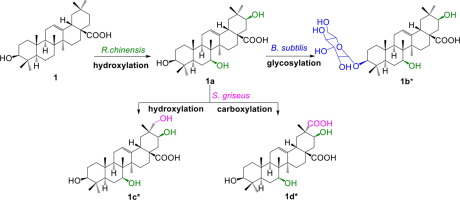
Tandem whole-cell biotransformation was applied successfully to deliver novel pentacyclic triterpenoid derivatives for the first time. In this process, the starting substrate oleanolic acid (1) was biotransformed into a hydroxylated metabolite 1a by Rhizopus chinensis CICC 40335 and then was further glycosylated to 1b by Bacillus subtilis ATCC 6633. Moreover, metabolite 1a was furtherly oxidized by Streptomyces griseus ATCC 13273 and generated two new derivatives as 1c and 1d. To validate the feasibility, tandem biotransformation of 18β-glycyrrhetinic acid (2) by R. chinensis and B. subtilis was also conducted and offered a glycosylated derivative (2c). Finally, the neuroprotective effects of the derivatives were assessed on neural injury PC12 cell model induced by cobalt chloride.
中文翻译:

串联生物转化在生物合成具有神经保护作用的新五环三萜衍生物中的应用。
串联全细胞生物转化成功应用于首次交付新型五环三萜衍生物。在此过程中,起始底物齐墩果酸(1)被中华根霉CICC 40335生物转化为羟基化代谢物1a,然后被枯草芽孢杆菌ATCC 6633进一步糖基化为1b。此外,代谢产物1a被灰链霉菌ATCC 13273和进一步氧化。生成了两个新的导数,分别为1c和1d。为了验证可行性,还进行了中华稻和枯草芽孢杆菌对18β-甘草次酸(2)的串联生物转化,并提供了糖基化衍生物(2c)。最后,评估了衍生物对氯化钴诱导的神经损伤PC12细胞模型的神经保护作用。
更新日期:2020-01-02
中文翻译:

串联生物转化在生物合成具有神经保护作用的新五环三萜衍生物中的应用。
串联全细胞生物转化成功应用于首次交付新型五环三萜衍生物。在此过程中,起始底物齐墩果酸(1)被中华根霉CICC 40335生物转化为羟基化代谢物1a,然后被枯草芽孢杆菌ATCC 6633进一步糖基化为1b。此外,代谢产物1a被灰链霉菌ATCC 13273和进一步氧化。生成了两个新的导数,分别为1c和1d。为了验证可行性,还进行了中华稻和枯草芽孢杆菌对18β-甘草次酸(2)的串联生物转化,并提供了糖基化衍生物(2c)。最后,评估了衍生物对氯化钴诱导的神经损伤PC12细胞模型的神经保护作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号