Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of the furin cleavage motif for HIV-1 trimeric envelope glycoprotein by intact LC-MS analysis.
Analyst ( IF 3.6 ) Pub Date : 2020-01-14 , DOI: 10.1039/c9an02098e Nicole A Schneck 1 , Vera B Ivleva 1 , Cindy X Cai 1 , Jonathan W Cooper 1 , Q Paula Lei 1
Analyst ( IF 3.6 ) Pub Date : 2020-01-14 , DOI: 10.1039/c9an02098e Nicole A Schneck 1 , Vera B Ivleva 1 , Cindy X Cai 1 , Jonathan W Cooper 1 , Q Paula Lei 1
Affiliation
|
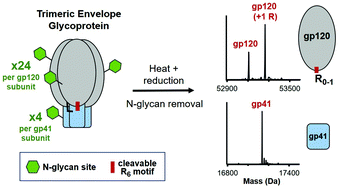
Generating a soluble and native-like trimeric envelope glycoprotein (Env) with high efficacy as an immunogen has been a major focus for developing an effective vaccine against HIV-1. The Env immunogen is a heavily glycosylated protein composed of 3 identical surface gp120 and gp41 subunits that form into a trimer of heterodimers (3 × 28 N-glycan sites). During Env immunogen production, endogenous furin works to cleave a hexa-arginine motif connecting the gp120 and gp41 subunits, which is needed to ensure proper protein folding and a native-like conformation of Env. Verification of the overall identity and proteolytic cleavage of Env is therefore important for HIV-1 vaccine development and product quality. Herein, we report the first work using LC-MS to (1) achieve fast and accurate intact mass measurement of Env after deglycosylation and (2) confidently identify the furin cleavage sites.
中文翻译:

通过完整的 LC-MS 分析表征 HIV-1 三聚体包膜糖蛋白的弗林蛋白酶切割基序。
生成具有高效免疫原的可溶性、类似天然的三聚体包膜糖蛋白 (Env) 一直是开发有效的 HIV-1 疫苗的主要焦点。 Env 免疫原是一种高度糖基化的蛋白质,由 3 个相同的表面 gp120 和 gp41 亚基组成,形成异二聚体三聚体(3 × 28 N-聚糖位点)。在 Env 免疫原生产过程中,内源性弗林蛋白酶会裂解连接 gp120 和 gp41 亚基的六精氨酸基序,这是确保适当的蛋白质折叠和 Env 的天然样构象所必需的。因此,Env 的整体身份和蛋白水解裂解的验证对于 HIV-1 疫苗的开发和产品质量非常重要。在此,我们报告了使用 LC-MS 的第一项工作,以 (1) 实现去糖基化后快速、准确的 Env 完整质量测量,以及 (2) 自信地识别弗林蛋白酶裂解位点。
更新日期:2020-03-03
中文翻译:

通过完整的 LC-MS 分析表征 HIV-1 三聚体包膜糖蛋白的弗林蛋白酶切割基序。
生成具有高效免疫原的可溶性、类似天然的三聚体包膜糖蛋白 (Env) 一直是开发有效的 HIV-1 疫苗的主要焦点。 Env 免疫原是一种高度糖基化的蛋白质,由 3 个相同的表面 gp120 和 gp41 亚基组成,形成异二聚体三聚体(3 × 28 N-聚糖位点)。在 Env 免疫原生产过程中,内源性弗林蛋白酶会裂解连接 gp120 和 gp41 亚基的六精氨酸基序,这是确保适当的蛋白质折叠和 Env 的天然样构象所必需的。因此,Env 的整体身份和蛋白水解裂解的验证对于 HIV-1 疫苗的开发和产品质量非常重要。在此,我们报告了使用 LC-MS 的第一项工作,以 (1) 实现去糖基化后快速、准确的 Env 完整质量测量,以及 (2) 自信地识别弗林蛋白酶裂解位点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号