当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface chemistry dictates stability and oxidation state of supported single metal catalyst atoms
Chemical Science ( IF 7.6 ) Pub Date : 2020/01/02 , DOI: 10.1039/c9sc05944j Konstantinos Alexopoulos 1 , Dionisios G Vlachos 1
Chemical Science ( IF 7.6 ) Pub Date : 2020/01/02 , DOI: 10.1039/c9sc05944j Konstantinos Alexopoulos 1 , Dionisios G Vlachos 1
Affiliation
|
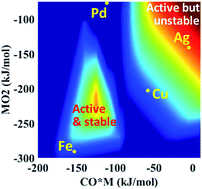
Single atom catalysts receive considerable attention due to reducing noble metal utilization and potentially eliminating certain side reactions. Yet, the rational design of highly reactive and stable single atom catalysts is hampered by the current lack of fundamental insights at the single atom limit. Here, density functional theory calculations are performed for a prototype reaction, namely CO oxidation, over different single metal atoms supported on alumina. The governing reaction mechanisms and scaling relations are identified using microkinetic modeling and principal component analysis, respectively. A large change in the oxophilicity of the supported single metal atom leads to changes in the rate-determining step and the catalyst resting state. Multi-response surfaces are introduced and built cheaply using a descriptor-based, closed form kinetic model to describe simultaneously the activity, stability, and oxidation state of single metal atom catalysts. A double peaked volcano in activity is observed due to competing rate-determining steps and catalytic cycles. Reaction orders of reactants provide excellent kinetic signatures of the catalyst state. Importantly, the surface chemistry determines the stability, oxidation, and resting state of the catalyst.
中文翻译:

表面化学决定了负载单金属催化剂原子的稳定性和氧化态
由于减少了贵金属的利用率并可能消除某些副反应,单原子催化剂受到了相当多的关注。然而,由于目前缺乏对单原子极限的基本认识,高反应性和稳定的单原子催化剂的合理设计受到阻碍。在这里,对氧化铝上负载的不同单金属原子的原型反应(即 CO 氧化)进行密度泛函理论计算。分别使用微动力学建模和主成分分析来确定控制反应机制和比例关系。负载的单金属原子亲氧性的巨大变化导致限速步骤和催化剂静止状态的变化。使用基于描述符的封闭式动力学模型引入并廉价构建多响应表面,以同时描述单金属原子催化剂的活性、稳定性和氧化态。由于竞争的速率决定步骤和催化循环,观察到活动中的双峰火山。反应物的反应级数提供了催化剂状态的良好动力学特征。重要的是,表面化学决定了催化剂的稳定性、氧化和静止状态。
更新日期:2020-02-13
中文翻译:

表面化学决定了负载单金属催化剂原子的稳定性和氧化态
由于减少了贵金属的利用率并可能消除某些副反应,单原子催化剂受到了相当多的关注。然而,由于目前缺乏对单原子极限的基本认识,高反应性和稳定的单原子催化剂的合理设计受到阻碍。在这里,对氧化铝上负载的不同单金属原子的原型反应(即 CO 氧化)进行密度泛函理论计算。分别使用微动力学建模和主成分分析来确定控制反应机制和比例关系。负载的单金属原子亲氧性的巨大变化导致限速步骤和催化剂静止状态的变化。使用基于描述符的封闭式动力学模型引入并廉价构建多响应表面,以同时描述单金属原子催化剂的活性、稳定性和氧化态。由于竞争的速率决定步骤和催化循环,观察到活动中的双峰火山。反应物的反应级数提供了催化剂状态的良好动力学特征。重要的是,表面化学决定了催化剂的稳定性、氧化和静止状态。











































 京公网安备 11010802027423号
京公网安备 11010802027423号