当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Characterization of a Paclitaxel Formulation Activated by Extracellular MMP9.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.bioconjchem.9b00865 Daniel Ehrsam 1 , Sandro Sieber 2 , Mouhssin Oufir 3 , Fabiola Porta 1 , Matthias Hamburger 3 , Jörg Huwyler 2 , Henriette E Meyer Zu Schwabedissen 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.bioconjchem.9b00865 Daniel Ehrsam 1 , Sandro Sieber 2 , Mouhssin Oufir 3 , Fabiola Porta 1 , Matthias Hamburger 3 , Jörg Huwyler 2 , Henriette E Meyer Zu Schwabedissen 1
Affiliation
|
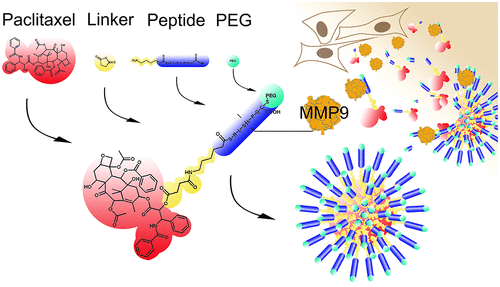
The concept of triggered drug release offers a possibility to overcome the toxic side effects of chemotherapeutics in cancer treatment by reducing systemic exposure to the active drug. In the present work, the concept foresees the use of the extracellular enzyme MMP9 as an enzymatic trigger for drug release in the proximity of tumor cells.
METHODS
A paclitaxel-hemisuccinate-peptide conjugate as a building block for self-assembling nanoparticles was synthesized using standard conjugation approaches. The building block was purified via preparative HPLC and analyzed by LC-MS. Nanoparticles were formed using the nanoprecipitation method and characterized. For selection of a suitable in vitro model system, common bioanalytical methods were used to determine mRNA expression, enzyme amount, and activity of MMP9.
RESULTS
The MMP9-labile prodrug was synthesized and characterized. Nanoparticles were formed out of MMP9-labile conjugate-building blocks. The nanoparticle's diameter averaged at around 120 nm and presented a spherical shape. LN-18 cells, a glioblastoma multiforme derived cell line, were chosen as an in vitro model based on findings in cancer tissue and cell line characterization. The prodrug showed cytotoxicity in LN-18 cells, which was reduced by addition of an MMP9 inhibitor.
CONCLUSION
taken together, we confirmed increased MMP9 in several cancer tissues (cervical, esophageal, lung, and brain) compared to healthy tissue and showed the effectiveness of MMP9-labile prodrug in in vitro tests.
中文翻译:

由细胞外MMP9激活的紫杉醇制剂的设计,合成和表征。
触发药物释放的概念提供了一种可能性,即通过减少与活性药物的全身接触来克服化学疗法在癌症治疗中的毒副作用。在目前的工作中,该概念预见了将细胞外酶MMP9用作在肿瘤细胞附近释放药物的酶促触发剂。方法使用标准缀合方法合成了紫杉醇-半琥珀酸酯-肽共轭物,作为自组装纳米粒子的构建基块。该结构单元通过制备型HPLC纯化,并通过LC-MS分析。使用纳米沉淀方法形成纳米颗粒并进行表征。为了选择合适的体外模型系统,常用的生物分析方法用于确定mRNA表达,酶量和MMP9的活性。结果合成并表征了MMP9不稳定的前药。纳米颗粒由MMP9不稳定的共轭结构单元形成。纳米粒子的平均直径约为120 nm,呈球形。基于癌症组织和细胞系表征的发现,选择LN-18细胞(多形性胶质母细胞瘤来源的细胞系)作为体外模型。前药在LN-18细胞中显示出细胞毒性,通过添加MMP9抑制剂可降低这种毒性。综上所述,我们证实与健康组织相比,一些癌症组织(宫颈,食道,肺和脑)的MMP9含量增加,并在体外试验中显示了MMP9不稳定的前药的有效性。直径平均在120nm左右,呈球形。基于癌症组织和细胞系表征的发现,选择LN-18细胞(多形性胶质母细胞瘤来源的细胞系)作为体外模型。前药在LN-18细胞中显示出细胞毒性,通过添加MMP9抑制剂可降低这种毒性。综上所述,我们证实与健康组织相比,一些癌症组织(宫颈,食道,肺和脑)的MMP9含量增加,并在体外试验中显示了MMP9不稳定的前药的有效性。直径平均在120nm左右,呈球形。基于癌症组织和细胞系表征的发现,选择LN-18细胞(多形性胶质母细胞瘤来源的细胞系)作为体外模型。前药在LN-18细胞中显示出细胞毒性,通过添加MMP9抑制剂可降低这种毒性。综上所述,我们证实与健康组织相比,一些癌症组织(宫颈,食道,肺和脑)的MMP9含量增加,并在体外试验中显示了MMP9不稳定的前药的有效性。
更新日期:2020-01-10
中文翻译:

由细胞外MMP9激活的紫杉醇制剂的设计,合成和表征。
触发药物释放的概念提供了一种可能性,即通过减少与活性药物的全身接触来克服化学疗法在癌症治疗中的毒副作用。在目前的工作中,该概念预见了将细胞外酶MMP9用作在肿瘤细胞附近释放药物的酶促触发剂。方法使用标准缀合方法合成了紫杉醇-半琥珀酸酯-肽共轭物,作为自组装纳米粒子的构建基块。该结构单元通过制备型HPLC纯化,并通过LC-MS分析。使用纳米沉淀方法形成纳米颗粒并进行表征。为了选择合适的体外模型系统,常用的生物分析方法用于确定mRNA表达,酶量和MMP9的活性。结果合成并表征了MMP9不稳定的前药。纳米颗粒由MMP9不稳定的共轭结构单元形成。纳米粒子的平均直径约为120 nm,呈球形。基于癌症组织和细胞系表征的发现,选择LN-18细胞(多形性胶质母细胞瘤来源的细胞系)作为体外模型。前药在LN-18细胞中显示出细胞毒性,通过添加MMP9抑制剂可降低这种毒性。综上所述,我们证实与健康组织相比,一些癌症组织(宫颈,食道,肺和脑)的MMP9含量增加,并在体外试验中显示了MMP9不稳定的前药的有效性。直径平均在120nm左右,呈球形。基于癌症组织和细胞系表征的发现,选择LN-18细胞(多形性胶质母细胞瘤来源的细胞系)作为体外模型。前药在LN-18细胞中显示出细胞毒性,通过添加MMP9抑制剂可降低这种毒性。综上所述,我们证实与健康组织相比,一些癌症组织(宫颈,食道,肺和脑)的MMP9含量增加,并在体外试验中显示了MMP9不稳定的前药的有效性。直径平均在120nm左右,呈球形。基于癌症组织和细胞系表征的发现,选择LN-18细胞(多形性胶质母细胞瘤来源的细胞系)作为体外模型。前药在LN-18细胞中显示出细胞毒性,通过添加MMP9抑制剂可降低这种毒性。综上所述,我们证实与健康组织相比,一些癌症组织(宫颈,食道,肺和脑)的MMP9含量增加,并在体外试验中显示了MMP9不稳定的前药的有效性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号