当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Studies on Rh-Catalyzed Cycloisomerization of Homopropargylallene-Alkynes through C(sp3)–C(sp) Bond Activation
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-14 , DOI: 10.1021/acscatal.9b04985 Ying Ren 1, 2 , Zhenyang Lin 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-14 , DOI: 10.1021/acscatal.9b04985 Ying Ren 1, 2 , Zhenyang Lin 1
Affiliation
|
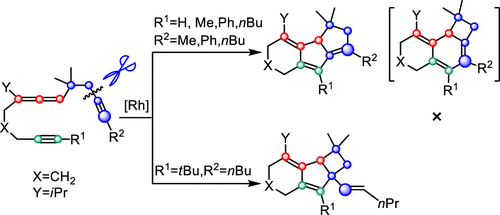
A systematic theoretical study has been carried out with the aid of density functional theory (DFT) calculations on the mechanism of the Rh-catalyzed cycloisomerization of homopropargylallene-alkynes through C(sp3)–C(sp) bond activation. The DFT results indicate that the catalytic cycle is divided into four main processes involving oxidative coupling, insertion, 1,3-alkyl migration, and 1,2-alkyl migration or 1,2-H migration. It is found that a Rh(I) Fischer carbene intermediate, which is derived from the third process 1,3-alkyl migration, selectively determines the formation of the six/five/five tricycle Product 1 or the six/five/four tricycle Product 2, depending on the substitutes on the substrates. The origin of cycloisomerization product selectivity has also been addressed.
中文翻译:

通过C(sp 3)–C(sp)键活化Rh催化同炔丙基烯丙炔的环异构化的理论研究
借助密度泛函理论(DFT)计算,通过C(sp 3)–C(sp)键活化,Rho催化高炔丙炔烯炔的环异构化机理进行了系统的理论研究。DFT结果表明,催化循环分为四个主要过程,涉及氧化偶联,插入,1,3-烷基迁移和1,2-烷基迁移或1,2-H迁移。发现源自第三过程1,3-烷基迁移的Rh(I)费歇尔卡宾中间体选择性地确定六/五/五三轮车产物1或六/五/四三轮车产物的形成。 2个,取决于基材上的替代物。还解决了环异构化产物选择性的起源。
更新日期:2020-01-15
中文翻译:

通过C(sp 3)–C(sp)键活化Rh催化同炔丙基烯丙炔的环异构化的理论研究
借助密度泛函理论(DFT)计算,通过C(sp 3)–C(sp)键活化,Rho催化高炔丙炔烯炔的环异构化机理进行了系统的理论研究。DFT结果表明,催化循环分为四个主要过程,涉及氧化偶联,插入,1,3-烷基迁移和1,2-烷基迁移或1,2-H迁移。发现源自第三过程1,3-烷基迁移的Rh(I)费歇尔卡宾中间体选择性地确定六/五/五三轮车产物1或六/五/四三轮车产物的形成。 2个,取决于基材上的替代物。还解决了环异构化产物选择性的起源。










































 京公网安备 11010802027423号
京公网安备 11010802027423号