当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidating the thermal and polymerization behaviours of benzoxazines from lignin derivatives
European Polymer Journal ( IF 5.8 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.eurpolymj.2019.109468 Acerina Trejo-Machin , Antoine Adjaoud , Laura Puchot , Reiner Dieden , Pierre Verge
European Polymer Journal ( IF 5.8 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.eurpolymj.2019.109468 Acerina Trejo-Machin , Antoine Adjaoud , Laura Puchot , Reiner Dieden , Pierre Verge
|
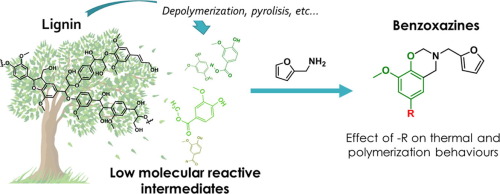
Abstract Due to their abundance, biophenols are widely targeted for the design of polybenzoxazines. Among them, ortho-methoxy substituted phenolic compounds are an attractive source of inspiration, abundantly issued from polyphenol depolymerisation. In most of the cases, they are substituted in the para-position with a group affecting the thermal properties of benzoxazine. This paper challenged to elucidate the role of the p-substituents found in lignin-occurring phenolic compounds, by studying the properties of purified monomers prepared from a solvent-free condensation of furfurylamine and model molecules: vanillin (V-fa), vanillic acid (VA-fa), guaiacol (G-fa), methyl vanillate (MV-fa) and creosol (Cr-fa). G-fa and V-fa were able to form 3D networks, the first one due to its unsubstituted para-position, and the second one due to the involvement of the –CHO group during the polymerization. Interestingly, these two substituents were providing a substantial stability to the monomers upon heating. On the contrary, Cr-fa and MV-fa, which were respectively dimerizing and oligomerizing, underwent substantially degradation during their curing.
中文翻译:

从木质素衍生物中阐明苯并恶嗪的热和聚合行为
摘要 由于生物酚含量丰富,因此被广泛用于设计聚苯并恶嗪。其中,邻甲氧基取代的酚类化合物是一个有吸引力的灵感来源,大量来自多酚解聚。在大多数情况下,它们在对位被一个影响苯并恶嗪热性能的基团取代。本文旨在通过研究由糠胺和模型分子的无溶剂缩合制备的纯化单体的性质:香草醛 (V-fa)、香草酸 ( VA-fa)、愈创木酚 (G-fa)、香草酸甲酯 (MV-fa) 和甲酚 (Cr-fa)。G-fa 和 V-fa 能够形成 3D 网络,第一个由于其未取代的对位,第二个是由于聚合过程中 -CHO 基团的参与。有趣的是,这两个取代基在加热时为单体提供了显着的稳定性。相反,分别进行二聚化和低聚化的 Cr-fa 和 MV-fa 在固化过程中经历了实质性的降解。
更新日期:2020-02-01
中文翻译:

从木质素衍生物中阐明苯并恶嗪的热和聚合行为
摘要 由于生物酚含量丰富,因此被广泛用于设计聚苯并恶嗪。其中,邻甲氧基取代的酚类化合物是一个有吸引力的灵感来源,大量来自多酚解聚。在大多数情况下,它们在对位被一个影响苯并恶嗪热性能的基团取代。本文旨在通过研究由糠胺和模型分子的无溶剂缩合制备的纯化单体的性质:香草醛 (V-fa)、香草酸 ( VA-fa)、愈创木酚 (G-fa)、香草酸甲酯 (MV-fa) 和甲酚 (Cr-fa)。G-fa 和 V-fa 能够形成 3D 网络,第一个由于其未取代的对位,第二个是由于聚合过程中 -CHO 基团的参与。有趣的是,这两个取代基在加热时为单体提供了显着的稳定性。相反,分别进行二聚化和低聚化的 Cr-fa 和 MV-fa 在固化过程中经历了实质性的降解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号