当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Method to Calculate the Relative Binding Free Energy Differences of α-Helical Stapled Peptides.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-01 , DOI: 10.1021/acs.joc.9b03067 Pedro A Valiente 1 , David Becerra 1 , Philip M Kim 1, 2, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-01 , DOI: 10.1021/acs.joc.9b03067 Pedro A Valiente 1 , David Becerra 1 , Philip M Kim 1, 2, 3
Affiliation
|
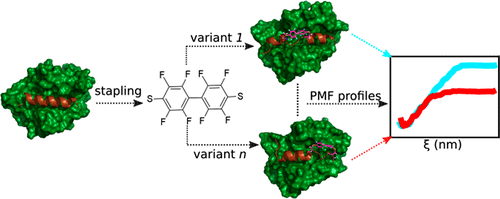
Hydrocarbon-stapled peptides are a class of bioactive α-helical ligands developed to target protein-protein interactions. Peptide stapling has benefited from the development of several chemical reactions to modulate their membrane permeability and binding affinity. However, in most current programs, choosing the best stapling positions is usually a trial-and-error process. Here, we develop a protocol to obtain optimal stapling positions computationally. Our method is based on molecular dynamics simulations and free energy calculations with nonequilibrium approaches; here, we predict the binding poses, hot-spot residues, and binding affinity differences of a set of perfluoroarene stapled α-helical peptides of the BIM BH3 peptide to the BCLXL receptor. The prediction of the hot-spot residues within the target peptide through computational alanine scanning anticipates not only the key residues for the receptor-peptide complex formation but also which positions should be avoided when applying the stapling groups. The staple moieties introduce local conformational changes not only in the replaced positions but also on their neighbor residues of the template peptide further affecting their binding behavior. Our approach is successful at rank-ordering the binding affinities of these stapled peptides with respect to the BIM BH3 peptide.
中文翻译:

一种计算α-螺旋钉合肽的相对结合自由能差的方法。
碳氢化合物肽是一类具有生物活性的α-螺旋配体,旨在靶向蛋白质-蛋白质相互作用。肽装订得益于几种化学反应的发展,以调节其膜通透性和结合亲和力。但是,在大多数当前程序中,选择最佳装订位置通常是一个反复试验的过程。在这里,我们开发了一种协议,可以通过计算获得最佳的装订位置。我们的方法基于分子动力学模拟和非平衡方法的自由能计算;在这里,我们预测BIM BH3肽的一组全氟芳烃钉合的α-螺旋肽与BCLXL受体的结合姿势,热点残基和结合亲和力差异。通过计算丙氨酸扫描预测目标肽内热点残基,不仅可以预测受体-肽复合物形成的关键残基,而且还可以预见在应用缝合基团时应避免的位置。短链部分不仅在取代的位置而且还在模板肽的它们的相邻残基上引入局部构象变化,这进一步影响了它们的结合行为。我们的方法成功地对这些钉合肽相对于BIM BH3肽的结合亲和力进行了排序。短链部分不仅在取代的位置而且还在模板肽的它们的相邻残基上引入局部构象变化,这进一步影响了它们的结合行为。我们的方法成功地对这些钉合肽相对于BIM BH3肽的结合亲和力进行了排序。短链部分不仅在取代的位置而且还在模板肽的它们的相邻残基上引入局部构象变化,这进一步影响了它们的结合行为。我们的方法成功地对这些钉合肽相对于BIM BH3肽的结合亲和力进行了排序。
更新日期:2020-01-13
中文翻译:

一种计算α-螺旋钉合肽的相对结合自由能差的方法。
碳氢化合物肽是一类具有生物活性的α-螺旋配体,旨在靶向蛋白质-蛋白质相互作用。肽装订得益于几种化学反应的发展,以调节其膜通透性和结合亲和力。但是,在大多数当前程序中,选择最佳装订位置通常是一个反复试验的过程。在这里,我们开发了一种协议,可以通过计算获得最佳的装订位置。我们的方法基于分子动力学模拟和非平衡方法的自由能计算;在这里,我们预测BIM BH3肽的一组全氟芳烃钉合的α-螺旋肽与BCLXL受体的结合姿势,热点残基和结合亲和力差异。通过计算丙氨酸扫描预测目标肽内热点残基,不仅可以预测受体-肽复合物形成的关键残基,而且还可以预见在应用缝合基团时应避免的位置。短链部分不仅在取代的位置而且还在模板肽的它们的相邻残基上引入局部构象变化,这进一步影响了它们的结合行为。我们的方法成功地对这些钉合肽相对于BIM BH3肽的结合亲和力进行了排序。短链部分不仅在取代的位置而且还在模板肽的它们的相邻残基上引入局部构象变化,这进一步影响了它们的结合行为。我们的方法成功地对这些钉合肽相对于BIM BH3肽的结合亲和力进行了排序。短链部分不仅在取代的位置而且还在模板肽的它们的相邻残基上引入局部构象变化,这进一步影响了它们的结合行为。我们的方法成功地对这些钉合肽相对于BIM BH3肽的结合亲和力进行了排序。










































 京公网安备 11010802027423号
京公网安备 11010802027423号