当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
EPR spectroscopy of iron- and nickel-doped [ZnAl]-layered double hydroxides: modeling active sites in heterogeneous water oxidation catalysts
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-12-31 , DOI: 10.1021/jacs.9b10273 Richard I Sayler 1 , Bryan M Hunter 1 , Wen Fu 1 , Harry B Gray 2 , R David Britt 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-12-31 , DOI: 10.1021/jacs.9b10273 Richard I Sayler 1 , Bryan M Hunter 1 , Wen Fu 1 , Harry B Gray 2 , R David Britt 1
Affiliation
|
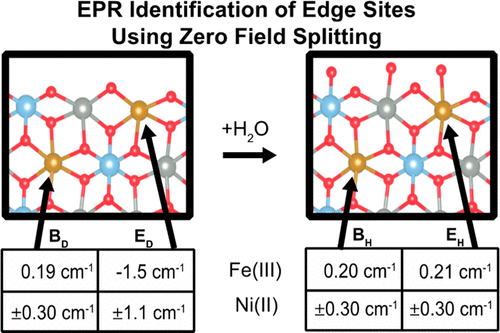
Iron-doped nickel layered double hydroxides (LDHs) are among the most active heterogeneous water oxidation catalysts. Due to inter-spin interactions, however, the high density of magnetic centers results in line-broadening in magnetic resonance spectra. As a result, gaining atomic-level insight into the catalytic mechanism via electron paramagnetic resonance (EPR) is not generally possible. To circumvent spin-spin broadening, iron and nickel atoms were doped into non-magnetic [ZnAl]-LDH materials and the coordination environments of the isolated Fe(III) and Ni(II) sites were characterized. Multifrequency EPR spectroscopy identified two distinct Fe(III) sites (S = 5/2) in [Fe:ZnAl]-LDH. Changes in zero field splitting (ZFS) were induced by dehydration of the material, revealing that one of the Fe(III) sites is solvent-exposed (i.e. at an edge, corner, or defect site). These solvent-exposed sites feature an axial ZFS of 0.21 cm-1 when hydrated. The ZFS increases dramatically upon dehydration (to -1.5 cm-1), owing to lower symmetry and a decrease in the coordination number of iron. The ZFS of the other ("inert") Fe(III) site maintains an axial ZFS of 0.19-0.20 cm-1 under both hydrated and dehydrated conditions. We observed a similar effect in [Ni:ZnAl]-LDH materials; notably, Ni(II) (S = 1) atoms displayed a single, small ZFS (±0.30 cm-1) in hydrated material, whereas two distinct Ni(II) ZFS values (±0.30 and ±1.1 cm-1) were observed in the dehydrated samples. Although the magnetically-dilute materials were not active catalysts, the identification of model sites in which the coordination environments of iron and nickel were particularly labile (e.g. by simple vacuum drying) is an important step towards identifying sites in which the coordination number may drop spontaneously in water, a probable mechanism of water oxidation in functional materials.
中文翻译:

铁和镍掺杂的 [ZnAl] 层状双氢氧化物的 EPR 光谱:模拟多相水氧化催化剂中的活性位点
铁掺杂镍层状双氢氧化物 (LDH) 是最活跃的多相水氧化催化剂之一。然而,由于自旋间相互作用,磁中心的高密度导致磁共振谱线变宽。因此,通过电子顺磁共振 (EPR) 获得对催化机制的原子级洞察通常是不可能的。为了避免自旋-自旋展宽,铁和镍原子被掺杂到非磁性 [ZnAl]-LDH 材料中,并表征了孤立的 Fe(III) 和 Ni(II) 位点的配位环境。多频 EPR 光谱在 [Fe:ZnAl]-LDH 中鉴定了两个不同的 Fe(III) 位点 (S = 5/2)。零场分裂 (ZFS) 的变化是由材料脱水引起的,这表明 Fe(III) 位点之一是溶剂暴露的(即在边缘,角或缺陷部位)。这些暴露于溶剂的部位在水合时具有 0.21 cm-1 的轴向 ZFS。由于较低的对称性和铁的配位数减少,脱水后 ZFS 显着增加(至 -1.5 cm-1)。另一个(“惰性”)Fe(III) 位点的 ZFS 在水合和脱水条件下均保持 0.19-0.20 cm-1 的轴向 ZFS。我们在 [Ni:ZnAl]-LDH 材料中观察到了类似的效果;值得注意的是,Ni(II) (S = 1) 原子在水合材料中显示出单个小的 ZFS (±0.30 cm-1),而观察到两个不同的 Ni(II) ZFS 值(±0.30 和 ±1.1 cm-1)在脱水样品中。虽然磁性稀释材料不是活性催化剂,但识别铁和镍的配位环境特别不稳定的模型位点(例如
更新日期:2019-12-31
中文翻译:

铁和镍掺杂的 [ZnAl] 层状双氢氧化物的 EPR 光谱:模拟多相水氧化催化剂中的活性位点
铁掺杂镍层状双氢氧化物 (LDH) 是最活跃的多相水氧化催化剂之一。然而,由于自旋间相互作用,磁中心的高密度导致磁共振谱线变宽。因此,通过电子顺磁共振 (EPR) 获得对催化机制的原子级洞察通常是不可能的。为了避免自旋-自旋展宽,铁和镍原子被掺杂到非磁性 [ZnAl]-LDH 材料中,并表征了孤立的 Fe(III) 和 Ni(II) 位点的配位环境。多频 EPR 光谱在 [Fe:ZnAl]-LDH 中鉴定了两个不同的 Fe(III) 位点 (S = 5/2)。零场分裂 (ZFS) 的变化是由材料脱水引起的,这表明 Fe(III) 位点之一是溶剂暴露的(即在边缘,角或缺陷部位)。这些暴露于溶剂的部位在水合时具有 0.21 cm-1 的轴向 ZFS。由于较低的对称性和铁的配位数减少,脱水后 ZFS 显着增加(至 -1.5 cm-1)。另一个(“惰性”)Fe(III) 位点的 ZFS 在水合和脱水条件下均保持 0.19-0.20 cm-1 的轴向 ZFS。我们在 [Ni:ZnAl]-LDH 材料中观察到了类似的效果;值得注意的是,Ni(II) (S = 1) 原子在水合材料中显示出单个小的 ZFS (±0.30 cm-1),而观察到两个不同的 Ni(II) ZFS 值(±0.30 和 ±1.1 cm-1)在脱水样品中。虽然磁性稀释材料不是活性催化剂,但识别铁和镍的配位环境特别不稳定的模型位点(例如











































 京公网安备 11010802027423号
京公网安备 11010802027423号