Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multimodal Nanocarrier Probes Reveal Superior Biodistribution Quantification by Isotopic Analysis over Fluorescence.
ACS Nano ( IF 15.8 ) Pub Date : 2020-01-07 , DOI: 10.1021/acsnano.9b06504 Hongping Deng , Christian J Konopka , Tzu-Wen L Cross , Kelly S Swanson , Lawrence W Dobrucki 1 , Andrew M Smith 1
ACS Nano ( IF 15.8 ) Pub Date : 2020-01-07 , DOI: 10.1021/acsnano.9b06504 Hongping Deng , Christian J Konopka , Tzu-Wen L Cross , Kelly S Swanson , Lawrence W Dobrucki 1 , Andrew M Smith 1
Affiliation
|
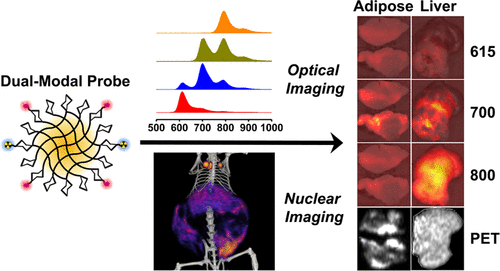
Absolute measurements of biodistribution are essential for understanding and optimizing the function of nanomaterials for in vivo diagnostic and therapeutic applications. Biodistribution analysis by optical imaging is desirable due to its low cost, wide accessibility, and high-throughput nature, but it is substantially less accurate than isotopic and chemical techniques. In this work, we developed multimodal probes for optical and nuclear imaging to analyze the quantitative limits of optical contrast in the red and near-infrared spectra for polysaccharide nanocarriers targeting macrophage cells. Probes incorporating three zwitterionic fluorophores together with radioactive copper distributed diffusely to optically dissimilar tissues that were either white (visceral adipose tissue) or dark red (liver and spleen) in obese rodents. We used in vivo positron emission tomography/computed tomography (PET/CT) imaging, in vivo hyperspectral tomographic fluorescence imaging, and ex vivo optical and isotopic analyses to determine correlations between optical and nuclear signals. PET imaging strongly correlated with standardized ex vivo methods for all tissue types, whereas no fluorescence signals exhibited substantial accuracy in quantification or localization in vivo. Optical imaging of resected tissues was most accurate in the 700 nm wavelength window, but only in white tissues. This work suggests that fluorescence can be used to measure diffuse probe distribution in white tissues over time or across animals, but not red tissues and not deep in the body. This work also highlights the importance of choosing validated experimental protocols and describes how optical measurements are impacted by fluorophore class and spectral properties, tissue properties, and imaging workflow.
中文翻译:

多模态纳米载体探针通过同位素分析显示优于荧光的生物分布定量。
生物分布的绝对测量对于理解和优化纳米材料在体内诊断和治疗应用中的功能至关重要。通过光学成像进行的生物分布分析因其成本低、可访问性广和高通量而受到人们的欢迎,但它的准确性远远低于同位素和化学技术。在这项工作中,我们开发了用于光学和核成像的多模态探针,以分析针对巨噬细胞的多糖纳米载体的红色和近红外光谱中光学对比度的定量极限。包含三个两性离子荧光团和放射性铜的探针分散分布到肥胖啮齿动物中光学不同的组织,这些组织要么是白色(内脏脂肪组织),要么是深红色(肝脏和脾脏)。我们使用体内正电子发射断层扫描/计算机断层扫描(PET/CT)成像、体内高光谱断层扫描荧光成像以及离体光学和同位素分析来确定光学信号和核信号之间的相关性。PET 成像与所有组织类型的标准化离体方法密切相关,而没有荧光信号在体内定量或定位方面表现出很高的准确性。切除组织的光学成像在 700 nm 波长窗口中最准确,但仅限于白色组织。这项工作表明,荧光可用于测量白色组织中随着时间的推移或在动物之间的扩散探针分布,但不能测量红色组织,也不能测量身体深处的探针分布。这项工作还强调了选择经过验证的实验方案的重要性,并描述了荧光团类别和光谱特性、组织特性和成像工作流程如何影响光学测量。
更新日期:2020-01-07
中文翻译:

多模态纳米载体探针通过同位素分析显示优于荧光的生物分布定量。
生物分布的绝对测量对于理解和优化纳米材料在体内诊断和治疗应用中的功能至关重要。通过光学成像进行的生物分布分析因其成本低、可访问性广和高通量而受到人们的欢迎,但它的准确性远远低于同位素和化学技术。在这项工作中,我们开发了用于光学和核成像的多模态探针,以分析针对巨噬细胞的多糖纳米载体的红色和近红外光谱中光学对比度的定量极限。包含三个两性离子荧光团和放射性铜的探针分散分布到肥胖啮齿动物中光学不同的组织,这些组织要么是白色(内脏脂肪组织),要么是深红色(肝脏和脾脏)。我们使用体内正电子发射断层扫描/计算机断层扫描(PET/CT)成像、体内高光谱断层扫描荧光成像以及离体光学和同位素分析来确定光学信号和核信号之间的相关性。PET 成像与所有组织类型的标准化离体方法密切相关,而没有荧光信号在体内定量或定位方面表现出很高的准确性。切除组织的光学成像在 700 nm 波长窗口中最准确,但仅限于白色组织。这项工作表明,荧光可用于测量白色组织中随着时间的推移或在动物之间的扩散探针分布,但不能测量红色组织,也不能测量身体深处的探针分布。这项工作还强调了选择经过验证的实验方案的重要性,并描述了荧光团类别和光谱特性、组织特性和成像工作流程如何影响光学测量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号