当前位置:
X-MOL 学术
›
Arab. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diazenyl schiff bases: Synthesis, spectral analysis, antimicrobial studies and cytotoxic activity on human colorectal carcinoma cell line (HCT-116)
Arabian Journal of Chemistry ( IF 5.3 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.arabjc.2017.05.004 Harmeet Kaur , Siong Meng Lim , Kalavathy Ramasamy , Mani Vasudevan , Syed Adnan Ali Shah , Balasubramanian Narasimhan
Arabian Journal of Chemistry ( IF 5.3 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.arabjc.2017.05.004 Harmeet Kaur , Siong Meng Lim , Kalavathy Ramasamy , Mani Vasudevan , Syed Adnan Ali Shah , Balasubramanian Narasimhan
|
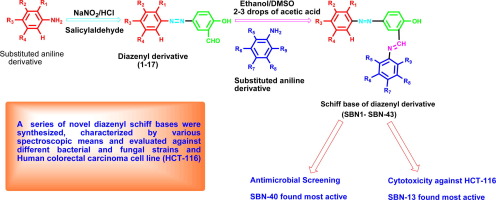
Abstract A series of diazenyl schiff bases have been synthesized by reaction of salicylaldehyde containing azo dyes with various substituted aniline derivatives in the presence of acetic acid as catalyst. The structures of diazenyl derivatives were determined by FTIR, UV–vis, 1H NMR, 13C NMR, CHN analysis, fluorimetric and mass spectroscopic studies. The synthesized derivatives were screened for their in vitro antimicrobial activity against various Gram-positive (S. aureus, B. subtilis, B. cereus), Gram-negative (S. typhi, S. enterica, E. coli, P. aeruginosa) bacterial and fungal (C. albicans, A. niger and A. fumigatus) strains, using cefadroxil (antibacterial) and fluconazole (antifungal) as standard drugs. The diazenyl schiff bases were also screened for their cytotoxicity against human colorectal carcinoma cell line (HCT-116) using 5-fluorouracil as standard drug by Sulforhodamine-B Stain (SRB) assay. The schiff bases exhibited significant activity toward both Gram-positive, Gram-negative bacterial and fungal strains. Most of the synthesized derivatives showed high activity against S. enterica. 4-((2,5-Dichlorophenyl)diazenyl)-2-((3-bromophenylimino)methyl)phenol (SBN-40) was found to be very active against S. aureus, B. cereus and E. coli, with MIC = 0.69 (µM/ml × 102). The compound 4-((2-bromophenyl)diazenyl)-2-((4-nitrophenylimino)methyl)phenol (SBN-13) possessed comparable activity (IC50 = 7.5 µg/ml) to the standard drug 5-fluorouracil (IC50 = 3.0 µg/ml) against human colorectal carcinoma cell line (HCT-116).
中文翻译:

二氮烯席夫碱:对人结肠直肠癌细胞系 (HCT-116) 的合成、光谱分析、抗菌研究和细胞毒活性
摘要 以含偶氮染料的水杨醛与各种取代苯胺衍生物在乙酸为催化剂下反应合成了一系列二氮烯席夫碱。二氮烯衍生物的结构通过 FTIR、UV-vis、1H NMR、13C NMR、CHN 分析、荧光和质谱研究确定。筛选合成的衍生物对各种革兰氏阳性菌(金黄色葡萄球菌、枯草芽孢杆菌、蜡状芽孢杆菌)、革兰氏阴性菌(伤寒沙门氏菌、肠道沙门氏菌、大肠杆菌、铜绿假单胞菌)的体外抗菌活性细菌和真菌(白色念珠菌、黑曲霉和烟曲霉)菌株,使用头孢羟氨苄(抗菌)和氟康唑(抗真菌)作为标准药物。还使用 5-氟尿嘧啶作为标准药物通过 Sulforhodamine-B 染色 (SRB) 试验筛选了二氮烯基希夫碱对人结肠直肠癌细胞系 (HCT-116) 的细胞毒性。席夫碱对革兰氏阳性、革兰氏阴性细菌和真菌菌株均表现出显着的活性。大多数合成的衍生物对 S. enterica 显示出高活性。4-((2,5-Dichlorophenyl)diazenyl)-2-((3-bromophenylimino)methyl)phenol (SBN-40) 被发现对金黄色葡萄球菌、蜡状芽孢杆菌和大肠杆菌非常有效,具有 MIC = 0.69 (µM/ml × 102)。化合物 4-((2-溴苯基)二氮烯基)-2-((4-硝基苯基亚氨基)甲基)苯酚 (SBN-13) 与标准药物 5-氟尿嘧啶 (IC50 = 7.5 µg/ml) 具有相当的活性 (IC50 = 7.5 µg/ml) 3.0 µg/ml) 对抗人结肠直肠癌细胞系 (HCT-116)。
更新日期:2020-01-01
中文翻译:

二氮烯席夫碱:对人结肠直肠癌细胞系 (HCT-116) 的合成、光谱分析、抗菌研究和细胞毒活性
摘要 以含偶氮染料的水杨醛与各种取代苯胺衍生物在乙酸为催化剂下反应合成了一系列二氮烯席夫碱。二氮烯衍生物的结构通过 FTIR、UV-vis、1H NMR、13C NMR、CHN 分析、荧光和质谱研究确定。筛选合成的衍生物对各种革兰氏阳性菌(金黄色葡萄球菌、枯草芽孢杆菌、蜡状芽孢杆菌)、革兰氏阴性菌(伤寒沙门氏菌、肠道沙门氏菌、大肠杆菌、铜绿假单胞菌)的体外抗菌活性细菌和真菌(白色念珠菌、黑曲霉和烟曲霉)菌株,使用头孢羟氨苄(抗菌)和氟康唑(抗真菌)作为标准药物。还使用 5-氟尿嘧啶作为标准药物通过 Sulforhodamine-B 染色 (SRB) 试验筛选了二氮烯基希夫碱对人结肠直肠癌细胞系 (HCT-116) 的细胞毒性。席夫碱对革兰氏阳性、革兰氏阴性细菌和真菌菌株均表现出显着的活性。大多数合成的衍生物对 S. enterica 显示出高活性。4-((2,5-Dichlorophenyl)diazenyl)-2-((3-bromophenylimino)methyl)phenol (SBN-40) 被发现对金黄色葡萄球菌、蜡状芽孢杆菌和大肠杆菌非常有效,具有 MIC = 0.69 (µM/ml × 102)。化合物 4-((2-溴苯基)二氮烯基)-2-((4-硝基苯基亚氨基)甲基)苯酚 (SBN-13) 与标准药物 5-氟尿嘧啶 (IC50 = 7.5 µg/ml) 具有相当的活性 (IC50 = 7.5 µg/ml) 3.0 µg/ml) 对抗人结肠直肠癌细胞系 (HCT-116)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号