当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermophysical and volumetric properties of mixtures {carvacrol + ethanol} at several temperatures and atmospheric pressure
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jct.2019.106042 José F. Martínez-López , Juan I. Pardo , José S. Urieta , Ana M. Mainar
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jct.2019.106042 José F. Martínez-López , Juan I. Pardo , José S. Urieta , Ana M. Mainar
|
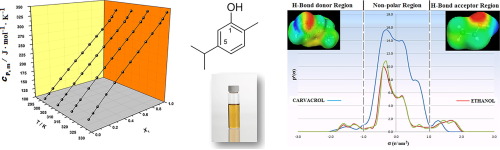
Abstract Experimental molar heat capacities at atmospheric pressure have been determined for the mixture {carvacrol + ethanol} every 10 K in the temperature interval (298.15–328.15) K and over the entire composition range with a Calvet type calorimeter. Densities, necessary for determining isobaric heat capacities, and ultrasonic speed of sound have been also measured at the same conditions. From these properties isobaric thermal expansivities, isentropic compressibilities, isothermal compressibilities, internal pressures and isochoric molar heat capacities have been calculated. Furthermore, in order to complete our thermophysical study the corresponding excess properties have been calculated and discussed in terms of molecular interactions. Finally, excess molar isobaric heat capacities have been calculated with COSMO-RS and compared to the experimental values.
中文翻译:

混合物 {香芹酚 + 乙醇} 在几个温度和大气压下的热物理和体积特性
摘要 使用 Calvet 型量热计在温度区间 (298.15–328.15) K 和整个组成范围内每 10 K 测定混合物 {香芹酚 + 乙醇} 在大气压下的实验摩尔热容。确定等压热容所需的密度和超声波声速也在相同条件下进行了测量。根据这些特性,等压热膨胀率、等熵压缩率、等温压缩率、内部压力和等容摩尔热容已被计算。此外,为了完成我们的热物理研究,已经根据分子相互作用计算和讨论了相应的过量特性。最后,
更新日期:2020-04-01
中文翻译:

混合物 {香芹酚 + 乙醇} 在几个温度和大气压下的热物理和体积特性
摘要 使用 Calvet 型量热计在温度区间 (298.15–328.15) K 和整个组成范围内每 10 K 测定混合物 {香芹酚 + 乙醇} 在大气压下的实验摩尔热容。确定等压热容所需的密度和超声波声速也在相同条件下进行了测量。根据这些特性,等压热膨胀率、等熵压缩率、等温压缩率、内部压力和等容摩尔热容已被计算。此外,为了完成我们的热物理研究,已经根据分子相互作用计算和讨论了相应的过量特性。最后,











































 京公网安备 11010802027423号
京公网安备 11010802027423号