当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into the active site and reaction mechanism for selective oxidation of methane to methanol using H2O2 on a Rh1/ZrO2 catalyst
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2019-12-31 , DOI: 10.1039/c9nj05667j Qi Zhao 1, 2, 3, 4, 5 , Bing Liu 1, 2, 3, 4, 5 , Yuebing Xu 1, 2, 3, 4, 5 , Feng Jiang 1, 2, 3, 4, 5 , Xiaohao Liu 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2019-12-31 , DOI: 10.1039/c9nj05667j Qi Zhao 1, 2, 3, 4, 5 , Bing Liu 1, 2, 3, 4, 5 , Yuebing Xu 1, 2, 3, 4, 5 , Feng Jiang 1, 2, 3, 4, 5 , Xiaohao Liu 1, 2, 3, 4, 5
Affiliation
|
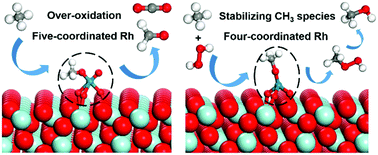
Direct methane conversion into value-added products has become increasingly important. However, it remains a great challenge to effectively activate methane and simultaneously suppress its over-oxidation. In this study, we performed a combined ab initio thermodynamics and DFT+U study to investigate the selective oxidation of methane to methanol on a ZrO2-supported Rh single-atom catalyst. The most preferred local environment of a Rh single atom was proposed according to the ab initio thermodynamics results. The DFT calculation results show that the five-coordinated Rh structure leads to the over-oxidation of CH3 species and thus prevents the formation of methanol. In contrast, the four-coordinated Rh can effectively stabilize the CH3 species by suppressing its further dehydrogenation. This is attributed to the fact that the geometric configuration of CH3 species at the four-coordinated Rh hinders the interaction between H in CH3 species and neighboring O. Two different methanol formation mechanisms at the four-coordinated Rh, namely the direct pathway and the CH3OOH intermediate pathway, were studied. It was found that the four-coordinated Rh facilitates the activation of H2O2 and the formation of CH3OOH, and thus the CH3OOH intermediate pathway plays a dominant role in methanol formation, in which CH3O species reacts with the OH group in H2O2 to form the CH3OOH intermediate and subsequently the deoxygenation of CH3OOH leads to the formation of methanol. This study provides atomic-scale insights into the active site and reaction mechanism for selective oxidation of methane to methanol on Rh1/ZrO2 catalysts.
中文翻译:

深入了解在Rh 1 / ZrO 2催化剂 上使用H 2 O 2将甲烷选择性氧化为甲醇的活性位点和反应机理
将甲烷直接转化为增值产品变得越来越重要。然而,有效地活化甲烷并同时抑制其过氧化仍然是巨大的挑战。在这项研究中,我们进行了从头算热力学和DFT + U的研究,以研究在ZrO 2负载的Rh单原子催化剂上甲烷选择性氧化为甲醇的过程。根据从头算热力学结果,提出了Rh单原子的最优选局部环境。DFT计算结果表明,五配位的Rh结构导致CH 3的过度氧化物种,从而防止甲醇的形成。相反,四配位的Rh可通过抑制其进一步的脱氢而有效地稳定CH 3种类。这归因于以下事实:四配位Rh上的CH 3物种的几何构型阻碍了CH 3物种中的H与邻近的O之间的相互作用。四配位Rh上的两种不同的甲醇形成机理,即直接途径和研究了CH 3 OOH中间途径。发现四配位的Rh促进了H 2 O 2的活化和CH 3 OOH的形成,从而促进了CH 3的形成。OOH中间体途径在甲醇形成中起主要作用,其中CH 3 O物种与H 2 O 2中的OH基反应形成CH 3 OOH中间体,随后CH 3 OOH脱氧导致甲醇的形成。这项研究为Rh 1 / ZrO 2催化剂上甲烷选择性氧化为甲醇的活性位点和反应机理提供了原子尺度的见解。
更新日期:2020-01-15
中文翻译:

深入了解在Rh 1 / ZrO 2催化剂 上使用H 2 O 2将甲烷选择性氧化为甲醇的活性位点和反应机理
将甲烷直接转化为增值产品变得越来越重要。然而,有效地活化甲烷并同时抑制其过氧化仍然是巨大的挑战。在这项研究中,我们进行了从头算热力学和DFT + U的研究,以研究在ZrO 2负载的Rh单原子催化剂上甲烷选择性氧化为甲醇的过程。根据从头算热力学结果,提出了Rh单原子的最优选局部环境。DFT计算结果表明,五配位的Rh结构导致CH 3的过度氧化物种,从而防止甲醇的形成。相反,四配位的Rh可通过抑制其进一步的脱氢而有效地稳定CH 3种类。这归因于以下事实:四配位Rh上的CH 3物种的几何构型阻碍了CH 3物种中的H与邻近的O之间的相互作用。四配位Rh上的两种不同的甲醇形成机理,即直接途径和研究了CH 3 OOH中间途径。发现四配位的Rh促进了H 2 O 2的活化和CH 3 OOH的形成,从而促进了CH 3的形成。OOH中间体途径在甲醇形成中起主要作用,其中CH 3 O物种与H 2 O 2中的OH基反应形成CH 3 OOH中间体,随后CH 3 OOH脱氧导致甲醇的形成。这项研究为Rh 1 / ZrO 2催化剂上甲烷选择性氧化为甲醇的活性位点和反应机理提供了原子尺度的见解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号