当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Single-step formulation of levodopa-based nanotheranostics - strategy for ultra-sensitive high longitudinal relaxivity MRI guided switchable therapeutics.
Biomaterials Science ( IF 6.6 ) Pub Date : 2020-01-22 , DOI: 10.1039/c9bm01799b Dan Tian 1 , Hongxia Xu 1 , Bing Xiao 1 , Xiaoxuan Zhou 2 , Xiangrui Liu 1 , Zhuxian Zhou 1 , Hirak K Patra 3 , Nigel Slater 3 , Jianbin Tang 1 , Youqing Shen 1
Biomaterials Science ( IF 6.6 ) Pub Date : 2020-01-22 , DOI: 10.1039/c9bm01799b Dan Tian 1 , Hongxia Xu 1 , Bing Xiao 1 , Xiaoxuan Zhou 2 , Xiangrui Liu 1 , Zhuxian Zhou 1 , Hirak K Patra 3 , Nigel Slater 3 , Jianbin Tang 1 , Youqing Shen 1
Affiliation
|
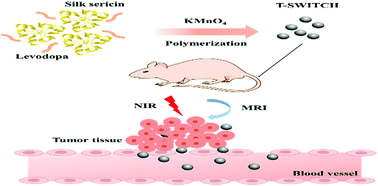
Nanotheranostics (combined diagnosis and therapy) is emerging as an integral part of future therapeutic strategies. However, the development and fabrication of a nanotheranostic module involves multistep processes and always faces formulation challenges. The complexity involved in its multi-step formulations hinders its reproducible industrial production and clinical translation. Therefore, a facile synthesis of multifunctional nanotheranostics is critical to its translational success. In this report, we have developed a one-pot facile strategy to prepare a MRI-visible photothermal theranostic switchable module (T-SWITCH). These nanoparticles are synthesized through polymerization of levodopa together with the reduction of KMnO4 in the presence of silk sericin for the formation of manganese dioxide particles within the T-SWITCH. The synthesized T-SWITCH showed a uniform size distribution of around 95.77 nm and high longitudinal relaxivity coefficient (r1) of up to 61.94 mM-1 s-1. The reported r1 of the T-SWITCH is exceedingly higher than that of any other previously reported manganese-based contrast agents with first-rate in vitro and in vivo contrast enhancement capability. The T-SWITCH can be activated to switch its therapeutic mode using near-infrared (NIR) light. It exhibited strong excitable absorption in the safer and biological NIR window between 650 and 900 nm. We have validated the significant anti-cancer therapeutic efficacy of T-SWITCH both in vitro and in vivo through switchable photothermal therapy.
中文翻译:

左旋多巴为基础的纳米治疗剂的单步配方-超敏感高纵向弛豫MRI指导的可切换治疗剂的策略。
纳米疗法(综合诊断和治疗)正在成为未来治疗策略不可或缺的一部分。然而,纳米热能模块的开发和制造涉及多步过程,并且总是面临配方方面的挑战。其多步骤配方涉及的复杂性阻碍了其可重现的工业生产和临床翻译。因此,多功能纳米热学的简便合成对其转化成功至关重要。在此报告中,我们已经开发了一种简单的策略来准备MRI可见光热疗治疗可转换模块(T-SWITCH)。这些纳米颗粒是通过左旋多巴的聚合以及在丝胶中存在的KMnO4的还原反应合成的,以在T-SWITCH中形成二氧化锰颗粒。合成的T-SWITCH显示出约95.77 nm的均匀尺寸分布和高达61.94 mM-1 s-1的高纵向弛豫系数(r1)。据报道,T-SWITCH的r1远远高于任何其他先前报道的具有一流的体外和体内对比增强能力的锰基造影剂。可以激活T-SWITCH以使用近红外(NIR)光切换其治疗模式。它在650至900 nm的更安全和生物学的NIR窗口中表现出强烈的兴奋性吸收。我们已经通过可切换的光热疗法在体内和体外验证了T-SWITCH的显着抗癌治疗效果。据报道,T-SWITCH的r1远远高于任何其他先前报道的具有一流的体外和体内对比度增强能力的锰基造影剂。可以激活T-SWITCH以使用近红外(NIR)光切换其治疗模式。它在650至900 nm的更安全和生物学的NIR窗口中表现出强烈的兴奋性吸收。我们已经通过可切换的光热疗法在体内和体外验证了T-SWITCH的显着抗癌治疗效果。据报道,T-SWITCH的r1远远高于任何其他先前报道的具有一流的体外和体内对比度增强能力的锰基造影剂。可以激活T-SWITCH以使用近红外(NIR)光切换其治疗模式。它在650至900 nm的更安全和生物学的NIR窗口中表现出强烈的兴奋性吸收。我们已经通过可切换的光热疗法在体内和体外验证了T-SWITCH的显着抗癌治疗效果。它在650至900 nm的更安全和生物学的NIR窗口中表现出强烈的兴奋性吸收。我们已经通过可切换的光热疗法在体内和体外验证了T-SWITCH的显着抗癌治疗效果。它在650至900 nm的更安全和生物学的NIR窗口中表现出强烈的兴奋性吸收。我们已经通过可切换的光热疗法在体内和体外验证了T-SWITCH的显着抗癌治疗效果。
更新日期:2020-03-19
中文翻译:

左旋多巴为基础的纳米治疗剂的单步配方-超敏感高纵向弛豫MRI指导的可切换治疗剂的策略。
纳米疗法(综合诊断和治疗)正在成为未来治疗策略不可或缺的一部分。然而,纳米热能模块的开发和制造涉及多步过程,并且总是面临配方方面的挑战。其多步骤配方涉及的复杂性阻碍了其可重现的工业生产和临床翻译。因此,多功能纳米热学的简便合成对其转化成功至关重要。在此报告中,我们已经开发了一种简单的策略来准备MRI可见光热疗治疗可转换模块(T-SWITCH)。这些纳米颗粒是通过左旋多巴的聚合以及在丝胶中存在的KMnO4的还原反应合成的,以在T-SWITCH中形成二氧化锰颗粒。合成的T-SWITCH显示出约95.77 nm的均匀尺寸分布和高达61.94 mM-1 s-1的高纵向弛豫系数(r1)。据报道,T-SWITCH的r1远远高于任何其他先前报道的具有一流的体外和体内对比增强能力的锰基造影剂。可以激活T-SWITCH以使用近红外(NIR)光切换其治疗模式。它在650至900 nm的更安全和生物学的NIR窗口中表现出强烈的兴奋性吸收。我们已经通过可切换的光热疗法在体内和体外验证了T-SWITCH的显着抗癌治疗效果。据报道,T-SWITCH的r1远远高于任何其他先前报道的具有一流的体外和体内对比度增强能力的锰基造影剂。可以激活T-SWITCH以使用近红外(NIR)光切换其治疗模式。它在650至900 nm的更安全和生物学的NIR窗口中表现出强烈的兴奋性吸收。我们已经通过可切换的光热疗法在体内和体外验证了T-SWITCH的显着抗癌治疗效果。据报道,T-SWITCH的r1远远高于任何其他先前报道的具有一流的体外和体内对比度增强能力的锰基造影剂。可以激活T-SWITCH以使用近红外(NIR)光切换其治疗模式。它在650至900 nm的更安全和生物学的NIR窗口中表现出强烈的兴奋性吸收。我们已经通过可切换的光热疗法在体内和体外验证了T-SWITCH的显着抗癌治疗效果。它在650至900 nm的更安全和生物学的NIR窗口中表现出强烈的兴奋性吸收。我们已经通过可切换的光热疗法在体内和体外验证了T-SWITCH的显着抗癌治疗效果。它在650至900 nm的更安全和生物学的NIR窗口中表现出强烈的兴奋性吸收。我们已经通过可切换的光热疗法在体内和体外验证了T-SWITCH的显着抗癌治疗效果。



























 京公网安备 11010802027423号
京公网安备 11010802027423号