Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitroglycerin for treatment of retained placenta: A randomised, placebo-controlled, multicentre, double-blind trial in the UK.
PLOS Medicine ( IF 10.5 ) Pub Date : 2019-12-30 , DOI: 10.1371/journal.pmed.1003001 Fiona C Denison 1 , Kathryn F Carruthers 1 , Jemma Hudson 2 , Gladys McPherson 2 , Gin Nie Chua 3 , Mathilde Peace 1 , Jane Brewin 4 , Nina Hallowell 5, 6 , Graham Scotland 2, 3 , Julia Lawton 7 , John Norrie 8 , Jane E Norman 1 ,
PLOS Medicine ( IF 10.5 ) Pub Date : 2019-12-30 , DOI: 10.1371/journal.pmed.1003001 Fiona C Denison 1 , Kathryn F Carruthers 1 , Jemma Hudson 2 , Gladys McPherson 2 , Gin Nie Chua 3 , Mathilde Peace 1 , Jane Brewin 4 , Nina Hallowell 5, 6 , Graham Scotland 2, 3 , Julia Lawton 7 , John Norrie 8 , Jane E Norman 1 ,
Affiliation
|
BACKGROUND
Retained placenta following vaginal delivery is a major cause of postpartum haemorrhage. Currently, the only effective treatments for a retained placenta are the surgical procedures of manual removal of placenta (MROP) and uterine curettage, which are not universally available, particularly in low- and middle-income countries. The objective of the trial was to determine whether sublingual nitroglycerin spray was clinically effective and cost-effective for medical treatment of retained placenta following vaginal delivery.
METHODS AND FINDINGS
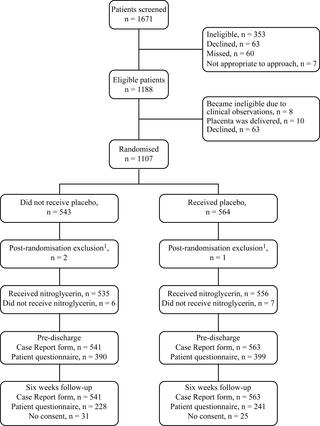
A randomised, placebo-controlled, double-blind trial was undertaken between October 2014 and July 2017 at 29 delivery units in the UK (Edinburgh, Glasgow, Manchester, Newcastle, Preston, Warrington, Chesterfield, Crewe, Durham, West Middlesex, Aylesbury, Furness, Southampton, Bolton, Sunderland, Oxford, Nottingham [2 units], Burnley, Chertsey, Stockton-on-Tees, Middlesborough, Chester, Darlington, York, Reading, Milton Keynes, Telford, Frimley). In total, 1,107 women with retained placenta following vaginal delivery were recruited. The intervention was self-administered 2 puffs of sublingual nitroglycerin (800 μg; intervention, N = 543) or placebo spray (control, N = 564). The primary clinical outcome was the need for MROP, assessed at 15 minutes following administration of the intervention. Analysis was based on the intention-to-treat principle. The primary safety outcome was measured blood loss between study drug administration and transfer to the postnatal ward or other clinical area. The primary patient-sided outcomes were satisfaction with treatment and side-effect profile, assessed by questionnaires pre-discharge and 6 weeks post-delivery. Secondary clinical outcomes were measured at 5 and 15 minutes after study drug administration and prior to hospital discharge. There was no statistically significant or clinically meaningful difference in need for MROP by 15 minutes (primary clinical outcome, 505 [93.3%] for nitroglycerin versus 518 [92.0%] for placebo, odds ratio [OR] 1.01 [95% CI 0.98-1.04], p = 0.393) or blood loss (<500 ml: nitroglycerin, 238 [44.3%], versus placebo, 249 [44.5%]; 500 ml-1,000 ml: nitroglycerin, 180 [33.5%], versus placebo, 224 [40.0%]; >1,000 ml: nitroglycerin, 119 [22.2%], versus placebo, 87 [15.5%]; ordinal OR 1.14 [95% CI 0.88-1.48], p = 0.314) or satisfaction with treatment (nitroglycerin, 288 [75.4%], versus placebo, 303 [78.1%]; OR 0.87 [95% CI 0.62-1.22], p = 0.411) or health service costs (mean difference [£] 55.3 [95% CI -199.20 to 309.79]). Palpitations following drug administration were reported more often in the nitroglycerin group (36 [9.8%] versus 15 [4.0%], OR 2.60 [95% CI 1.40-4.84], p = 0.003). There were 52 serious adverse events during the trial, with no statistically significant difference in likelihood between groups (nitroglycerin, 27 [5.0%], versus placebo, 26 [4.6%]; OR 1.13 [95% CI 0.54-2.38], p = 0.747). The main limitation of our study was the low return rate for the 6-week postnatal questionnaire. There were, however, no differences in questionnaire return rates between study groups or between women who did and did not have MROP, with the patient-reported use of outpatient and primary care services at 6 weeks accounting for only a small proportion (approximately 5%) of overall health service costs.
CONCLUSIONS
In this study, we found that nitroglycerin is neither clinically effective nor cost-effective as a medical treatment for retained placenta, and has increased side effects, suggesting it should not be used. Further research is required to identify an effective medical treatment for retained placenta to reduce the morbidity caused by this condition, particularly in low- and middle-income countries where surgical management is not available.
TRIAL REGISTRATION
ISRCTN.com ISRCTN88609453 ClinicalTrials.gov NCT02085213.
中文翻译:

硝酸甘油治疗保留胎盘:英国的一项随机,安慰剂对照,多中心,双盲试验。
背景技术阴道分娩后保留的胎盘是产后出血的主要原因。当前,保留胎盘的唯一有效治疗方法是手动去除胎盘(MROP)和刮宫术的手术程序,这在世界范围内并不普遍,特别是在低收入和中等收入国家。该试验的目的是确定舌下硝化甘油喷雾剂在阴道分娩后对保留胎盘的医学治疗是否在临床上有效且具有成本效益。方法与结果2014年10月至2017年7月,在英国的29个分娩单位(爱丁堡,格拉斯哥,曼彻斯特,纽卡斯尔,普雷斯顿,沃灵顿,切斯特菲尔德,克鲁,达勒姆,西米德塞克斯)进行了一项随机,安慰剂对照,双盲试验,艾尔斯伯里,菲尼斯,南安普敦,博尔顿,桑德兰,牛津,诺丁汉[2个单位],伯恩利,彻特西,蒂斯托克顿,米德尔斯堡,切斯特,达林顿,约克,雷丁,米尔顿凯恩斯,特尔福德,弗里姆利)。总共招募了1,107名在阴道分娩后保留胎盘的妇女。干预措施为自行服用2口舌下硝化甘油(800μg;干预措施,N = 543)或安慰剂喷雾剂(对照组,N = 564)。主要的临床结果是对MROP的需要,在进行干预后15分钟进行评估。分析基于意向性治疗原则。主要安全结果是在研究药物给药与转移至产后病房或其他临床区域之间的失血量。患者的主要预后是对治疗和副作用的满意程度,在出院前和分娩后6周通过问卷进行评估。在研究药物给药后和出院前5分钟和15分钟测量次要临床结局。到15分钟时,MROP的需求没有统计学上的显着差异或临床意义(主要临床结果,硝酸甘油为505 [93.3%],而安慰剂为518 [92.0%],优势比[OR] 1.01 [95%CI 0.98-1.04] ],p = 0.393)或失血(<500 ml:硝酸甘油238 [44.3%],而安慰剂为249 [44.5%]; 500 ml-1,000 ml:硝酸甘油180,[33.5%],而安慰剂为224 [43.5%]。 40.0%];> 1,000毫升:硝酸甘油119 [22.2%],而安慰剂87 [15.5%];序贯OR 1.14 [95%CI 0.88-1.48],p = 0.314)或治疗满意度(硝酸甘油288 [ 75.4%],而安慰剂则为303 [78.1%];或0.87 [95%CI 0.62-1.22],p = 0。411)或医疗服务费用(平均差[£] 55.3 [95%CI -199.20至309.79])。在硝酸甘油组中,给药后心的发生率更高(36 [9.8%]比15 [4.0%],或2.60 [95%CI 1.40-4.84],p = 0.003)。试验期间发生了52次严重不良事件,各组之间的可能性无统计学差异(硝酸甘油27 [5.0%],而安慰剂26 [4.6%];或1.13 [95%CI 0.54-2.38],p = 0.747)。我们研究的主要局限性是6周产后问卷的返回率低。但是,研究组之间或进行过和未进行过MROP的女性之间的问卷返回率没有差异,患者报告的在第6周使用门诊和初级保健服务的费用仅占整体卫生服务费用的一小部分(约5%)。结论在这项研究中,我们发现硝化甘油作为保留胎盘的药物既无临床疗效,也不具有成本效益,并且副作用增加,表明不宜使用。需要进一步的研究来确定保留胎盘的有效治疗方法,以减少由这种疾病引起的发病率,尤其是在无法进行手术治疗的中低收入国家。试用注册ISRCTN.com ISRCTN88609453 ClinicalTrials.gov NCT02085213。我们发现硝酸甘油作为保留胎盘的药物既无临床疗效,也不具有成本效益,并且副作用增加,提示不宜使用。需要进一步的研究来确定保留胎盘的有效治疗方法,以减少由这种疾病引起的发病率,尤其是在无法进行手术治疗的中低收入国家。试用注册ISRCTN.com ISRCTN88609453 ClinicalTrials.gov NCT02085213。我们发现硝酸甘油作为保留胎盘的药物既无临床疗效,也不具有成本效益,并且副作用增加,提示不宜使用。需要进一步的研究来确定保留胎盘的有效治疗方法,以减少由这种疾病引起的发病率,尤其是在无法进行手术治疗的中低收入国家。试用注册ISRCTN.com ISRCTN88609453 ClinicalTrials.gov NCT02085213。
更新日期:2020-01-14
中文翻译:

硝酸甘油治疗保留胎盘:英国的一项随机,安慰剂对照,多中心,双盲试验。
背景技术阴道分娩后保留的胎盘是产后出血的主要原因。当前,保留胎盘的唯一有效治疗方法是手动去除胎盘(MROP)和刮宫术的手术程序,这在世界范围内并不普遍,特别是在低收入和中等收入国家。该试验的目的是确定舌下硝化甘油喷雾剂在阴道分娩后对保留胎盘的医学治疗是否在临床上有效且具有成本效益。方法与结果2014年10月至2017年7月,在英国的29个分娩单位(爱丁堡,格拉斯哥,曼彻斯特,纽卡斯尔,普雷斯顿,沃灵顿,切斯特菲尔德,克鲁,达勒姆,西米德塞克斯)进行了一项随机,安慰剂对照,双盲试验,艾尔斯伯里,菲尼斯,南安普敦,博尔顿,桑德兰,牛津,诺丁汉[2个单位],伯恩利,彻特西,蒂斯托克顿,米德尔斯堡,切斯特,达林顿,约克,雷丁,米尔顿凯恩斯,特尔福德,弗里姆利)。总共招募了1,107名在阴道分娩后保留胎盘的妇女。干预措施为自行服用2口舌下硝化甘油(800μg;干预措施,N = 543)或安慰剂喷雾剂(对照组,N = 564)。主要的临床结果是对MROP的需要,在进行干预后15分钟进行评估。分析基于意向性治疗原则。主要安全结果是在研究药物给药与转移至产后病房或其他临床区域之间的失血量。患者的主要预后是对治疗和副作用的满意程度,在出院前和分娩后6周通过问卷进行评估。在研究药物给药后和出院前5分钟和15分钟测量次要临床结局。到15分钟时,MROP的需求没有统计学上的显着差异或临床意义(主要临床结果,硝酸甘油为505 [93.3%],而安慰剂为518 [92.0%],优势比[OR] 1.01 [95%CI 0.98-1.04] ],p = 0.393)或失血(<500 ml:硝酸甘油238 [44.3%],而安慰剂为249 [44.5%]; 500 ml-1,000 ml:硝酸甘油180,[33.5%],而安慰剂为224 [43.5%]。 40.0%];> 1,000毫升:硝酸甘油119 [22.2%],而安慰剂87 [15.5%];序贯OR 1.14 [95%CI 0.88-1.48],p = 0.314)或治疗满意度(硝酸甘油288 [ 75.4%],而安慰剂则为303 [78.1%];或0.87 [95%CI 0.62-1.22],p = 0。411)或医疗服务费用(平均差[£] 55.3 [95%CI -199.20至309.79])。在硝酸甘油组中,给药后心的发生率更高(36 [9.8%]比15 [4.0%],或2.60 [95%CI 1.40-4.84],p = 0.003)。试验期间发生了52次严重不良事件,各组之间的可能性无统计学差异(硝酸甘油27 [5.0%],而安慰剂26 [4.6%];或1.13 [95%CI 0.54-2.38],p = 0.747)。我们研究的主要局限性是6周产后问卷的返回率低。但是,研究组之间或进行过和未进行过MROP的女性之间的问卷返回率没有差异,患者报告的在第6周使用门诊和初级保健服务的费用仅占整体卫生服务费用的一小部分(约5%)。结论在这项研究中,我们发现硝化甘油作为保留胎盘的药物既无临床疗效,也不具有成本效益,并且副作用增加,表明不宜使用。需要进一步的研究来确定保留胎盘的有效治疗方法,以减少由这种疾病引起的发病率,尤其是在无法进行手术治疗的中低收入国家。试用注册ISRCTN.com ISRCTN88609453 ClinicalTrials.gov NCT02085213。我们发现硝酸甘油作为保留胎盘的药物既无临床疗效,也不具有成本效益,并且副作用增加,提示不宜使用。需要进一步的研究来确定保留胎盘的有效治疗方法,以减少由这种疾病引起的发病率,尤其是在无法进行手术治疗的中低收入国家。试用注册ISRCTN.com ISRCTN88609453 ClinicalTrials.gov NCT02085213。我们发现硝酸甘油作为保留胎盘的药物既无临床疗效,也不具有成本效益,并且副作用增加,提示不宜使用。需要进一步的研究来确定保留胎盘的有效治疗方法,以减少由这种疾病引起的发病率,尤其是在无法进行手术治疗的中低收入国家。试用注册ISRCTN.com ISRCTN88609453 ClinicalTrials.gov NCT02085213。











































 京公网安备 11010802027423号
京公网安备 11010802027423号