Journal of Solid State Chemistry ( IF 3.2 ) Pub Date : 2019-12-31 , DOI: 10.1016/j.jssc.2019.121165 Guido Cerri , Antonio Brundu
|
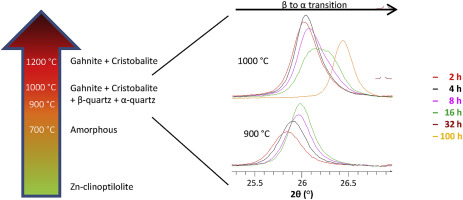
Zn-clinoptilolite was heated and analyzed ex-situ. Breakdown occurred between 400-700 °C. After 2 h at 850 °C gahnite and Zn-β-quartz nucleated. Exolution of zinc and aluminum from Zn-β-quartz determined Zn-α-quartz formation after 8 h. An increase in temperature and/or heating time determined exolution also in Zn-α-quartz, that progressively turned into cristobalite. Zn-β-quartz decreased over time at 1000 °C and disappeared after 100 h, whereas at 900 °C the quantitative ratio β-quartz/α-quartz asymptotically stabilized at ≈2.5. Rietveld refinements evidenced that at 900 °C the decrease of zinc occupancy followed an almost asymptotic path in both quartz phases. Considering the formula ZnxAl2xSi3-2xO6, if x < 0.173 ± 0.008 Zn-β-quartz is not stable at room temperature. Treatments of 2 h at 1100 and 1200 °C produced the same quantities of gahnite (≈20.6%) and glass (≈11.6%), and slight differences for cristobalite (56 vs. 63%) and quartz (8.4 vs. 1.5%). Basically, gahnite incorporated all Zn2+ contained in clinoptilolite.
中文翻译:

锌斜发沸石通过加热的固态转变
加热锌斜发沸石并进行非原位分析。击穿发生在400-700°C之间。在850°C下2小时后,钠长石和Zn-β-石英成核。从锌-β-石英中锌和铝的洗脱确定8小时后锌-α-石英的形成。温度和/或加热时间的增加也决定了Zn-α-石英中的溶出度,逐渐变成方石英。Zn-β-石英在1000°C时随时间下降,在100 h后消失,而在900°C时,β-石英/α-石英的定量比渐近稳定在≈2.5。Rietveld的精炼结果表明,在900°C时,两个石英相中锌的吸收率都遵循一条几乎渐近的路径。考虑公式Zn x Al 2x Si 3-2x O 6如果x <0.173±0.008Zn-β-石英在室温下不稳定。在1100和1200°C的温度下进行2小时的处理可产生相同量的针铁矿(≈20.6%)和玻璃(≈11.6%),方石英石(56 vs. 63%)和石英(8.4 vs. 1.5%)的细微差异。基本上,轻铁矿掺入斜发沸石中包含的所有Zn 2+。











































 京公网安备 11010802027423号
京公网安备 11010802027423号