当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Model for Counterion Binding and Charge Reversal on Protein Surfaces.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-12-30 , DOI: 10.1021/acs.molpharmaceut.9b01047 Jas Kalayan 1 , Richard H Henchman 1 , Jim Warwicker 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-12-30 , DOI: 10.1021/acs.molpharmaceut.9b01047 Jas Kalayan 1 , Richard H Henchman 1 , Jim Warwicker 1
Affiliation
|
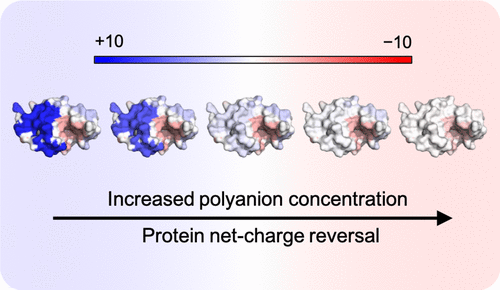
The structural stability and solubility of proteins in liquid therapeutic formulations is important, especially since new generations of therapeutics are designed for efficacy before consideration of stability. We introduce an electrostatic binding model to measure the net charge of proteins with bound ions in solution. The electrostatic potential on a protein surface is used to separately group together acidic and basic amino acids into patches, which are then iteratively bound with oppositely charged counterions. This model is aimed toward formulation chemists for initial screening of a range of conditions prior to lab-work. Computed results compare well with experimental zeta potential measurements from the literature covering a range of solution conditions. Importantly, the binding model reproduces the charge reversal phenomenon that is observed with polyvalent ion binding to proteins and its dependence on ion charge and concentration. Intriguingly, protein sequence can be used to give similarly good agreement with experiment as protein structure, interpreted as resulting from the close proximity of charged side chains on a protein surface. Further, application of the model to human proteins suggests that polyanion binding and overcharging, including charge reversal for cationic proteins, is a general feature. These results add to evidence that addition of polyanions to protein formulations could be a general mechanism for modulating solution stability.
中文翻译:

蛋白质表面抗衡离子结合和电荷反转的模型。
蛋白质在液体治疗制剂中的结构稳定性和溶解性很重要,尤其是因为新一代治疗剂是在考虑稳定性之前针对功效而设计的。我们引入静电结合模型来测量溶液中结合离子的蛋白质净电荷。蛋白质表面上的静电势用于将酸性氨基酸和碱性氨基酸分别分组为补丁,然后与带相反电荷的抗衡离子迭代结合。该模型面向配方化学家,用于在实验室工作之前对一系列条件进行初步筛选。计算结果与涵盖一系列溶液条件的文献中的实验zeta电位测量结果相当吻合。重要的,结合模型再现了电荷反转现象,该现象是通过多价离子与蛋白质结合及其对离子电荷和浓度的依赖性而观察到的。有趣的是,蛋白质序列可用于与蛋白质结构相似的实验,解释为蛋白质表面带电侧链的紧密接近所产生的结果。此外,将该模型应用于人蛋白质表明,聚阴离子结合和过度充电(包括阳离子蛋白质的电荷反转)是一个普遍特征。这些结果增加了证据,证明在蛋白质制剂中添加聚阴离子可能是调节溶液稳定性的一般机制。蛋白质序列可用于与实验产生相似的蛋白质结构一致性,解释为蛋白质表面带电侧链的紧密接近所产生的结果。此外,将该模型应用于人类蛋白质表明,聚阴离子的结合和过度充电(包括阳离子蛋白质的电荷逆转)是一个普遍特征。这些结果增加了证据,证明在蛋白质制剂中添加聚阴离子可能是调节溶液稳定性的一般机制。蛋白质序列可用于与实验产生相似的蛋白质结构一致性,解释为蛋白质表面带电侧链的紧密接近所产生的结果。此外,将该模型应用于人类蛋白质表明,聚阴离子的结合和过度充电(包括阳离子蛋白质的电荷逆转)是一个普遍特征。这些结果增加了证据,证明在蛋白质制剂中添加聚阴离子可能是调节溶液稳定性的一般机制。
更新日期:2020-01-09
中文翻译:

蛋白质表面抗衡离子结合和电荷反转的模型。
蛋白质在液体治疗制剂中的结构稳定性和溶解性很重要,尤其是因为新一代治疗剂是在考虑稳定性之前针对功效而设计的。我们引入静电结合模型来测量溶液中结合离子的蛋白质净电荷。蛋白质表面上的静电势用于将酸性氨基酸和碱性氨基酸分别分组为补丁,然后与带相反电荷的抗衡离子迭代结合。该模型面向配方化学家,用于在实验室工作之前对一系列条件进行初步筛选。计算结果与涵盖一系列溶液条件的文献中的实验zeta电位测量结果相当吻合。重要的,结合模型再现了电荷反转现象,该现象是通过多价离子与蛋白质结合及其对离子电荷和浓度的依赖性而观察到的。有趣的是,蛋白质序列可用于与蛋白质结构相似的实验,解释为蛋白质表面带电侧链的紧密接近所产生的结果。此外,将该模型应用于人蛋白质表明,聚阴离子结合和过度充电(包括阳离子蛋白质的电荷反转)是一个普遍特征。这些结果增加了证据,证明在蛋白质制剂中添加聚阴离子可能是调节溶液稳定性的一般机制。蛋白质序列可用于与实验产生相似的蛋白质结构一致性,解释为蛋白质表面带电侧链的紧密接近所产生的结果。此外,将该模型应用于人类蛋白质表明,聚阴离子的结合和过度充电(包括阳离子蛋白质的电荷逆转)是一个普遍特征。这些结果增加了证据,证明在蛋白质制剂中添加聚阴离子可能是调节溶液稳定性的一般机制。蛋白质序列可用于与实验产生相似的蛋白质结构一致性,解释为蛋白质表面带电侧链的紧密接近所产生的结果。此外,将该模型应用于人类蛋白质表明,聚阴离子的结合和过度充电(包括阳离子蛋白质的电荷逆转)是一个普遍特征。这些结果增加了证据,证明在蛋白质制剂中添加聚阴离子可能是调节溶液稳定性的一般机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号