当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanoporous Palladium Hydride for Electrocatalytic N2 Reduction under Ambient Conditions.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-12-30 , DOI: 10.1002/anie.201914335 Wence Xu 1 , Guilan Fan 1 , Jialiang Chen 2 , Jinhan Li 1 , Le Zhang 1 , Shengli Zhu 3 , Xuncheng Su 2 , Fangyi Cheng 1 , Jun Chen 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-12-30 , DOI: 10.1002/anie.201914335 Wence Xu 1 , Guilan Fan 1 , Jialiang Chen 2 , Jinhan Li 1 , Le Zhang 1 , Shengli Zhu 3 , Xuncheng Su 2 , Fangyi Cheng 1 , Jun Chen 1, 2
Affiliation
|
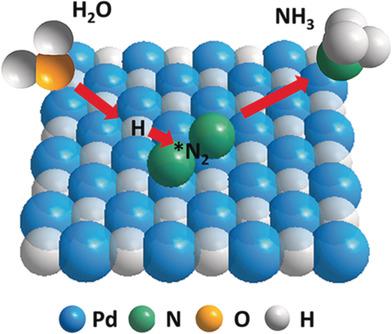
The electrocatalytic nitrogen reduction reaction (NRR) is an alternative eco-friendly strategy for sustainable N2 fixation with renewable energy. However, NRR suffers from sluggish kinetics owing to difficult N2 adsorption and N≡N cleavage. Now, nanoporous palladium hydride is reported as electrocatalyst for electrochemical N2 reduction under ambient conditions, achieving a high ammonia yield rate of 20.4 μg h-1 mg-1 with a Faradaic efficiency of 43.6 % at low overpotential of 150 mV. Isotopic hydrogen labeling studies suggest the involvement of lattice hydrogen atoms in the hydride as active hydrogen source. In situ Raman analysis and density functional theory (DFT) calculations further reveal the reduction of energy barrier for the rate-limiting *N2 H formation step. The unique protonation mode of palladium hydride would provide a new insight on designing efficient and robust electrocatalysts for nitrogen fixation.
中文翻译:

在环境条件下用于电催化还原N2的纳米多孔氢化钯。
电催化氮还原反应(NRR)是使用可再生能源可持续固氮的另一种环保策略。然而,由于难以吸收N2和N≡N裂解,NRR的动力学缓慢。现在,据报道纳米多孔氢化钯是在环境条件下用于电化学还原N2的电催化剂,在150mV的低超电势下,氨的产率高,达20.4μgh-1 mg-1,法拉第效率达43.6%。同位素氢标记研究表明,氢化物中的晶格氢原子作为活性氢源参与其中。原位拉曼分析和密度泛函理论(DFT)计算进一步揭示了限速* N2 H形成步骤能垒的降低。
更新日期:2020-01-24
中文翻译:

在环境条件下用于电催化还原N2的纳米多孔氢化钯。
电催化氮还原反应(NRR)是使用可再生能源可持续固氮的另一种环保策略。然而,由于难以吸收N2和N≡N裂解,NRR的动力学缓慢。现在,据报道纳米多孔氢化钯是在环境条件下用于电化学还原N2的电催化剂,在150mV的低超电势下,氨的产率高,达20.4μgh-1 mg-1,法拉第效率达43.6%。同位素氢标记研究表明,氢化物中的晶格氢原子作为活性氢源参与其中。原位拉曼分析和密度泛函理论(DFT)计算进一步揭示了限速* N2 H形成步骤能垒的降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号