Journal of Catalysis ( IF 6.5 ) Pub Date : 2019-12-31 , DOI: 10.1016/j.jcat.2019.12.022 Weiwei Wang , Zhenping Qu , Lixin Song , Qiang Fu
|
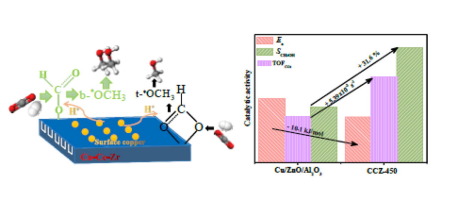
A series of ternary copper-cerium-zirconium catalysts containing two kinds of copper species, surface CuO and Cu-Ce-Zr solid solution, are prepared and studied for catalytic properties of CO2 hydrogenation to methanol. The copper-cerium- zirconium catalyst calcined at 450 °C (CCZ-450) is much more favorable for improving the nature of surface CuO species and forming Cu-Ce-Zr solid solution than others. The best catalytic behavior in terms of methanol selectivity (T = 280 °C, SCH3OH = 71.8%), turnover frequency (TOFCO2 = 13.4 × 10−2 s−1) and activation energy (Ea = 28.5 kJ/mol) are achieved using CCZ-450. The excellent catalytic performance of CCZ-450 is attributed to the stronger H2 adsorption ability arising from highly dispersed surface CuO specie with higher copper surface area and higher concentration of active bi/m-HCOO* intermediate caused by the formation of Cu-Ce-Zr solid solution. Both the dispersion and surface area of active sites and the activation abilities of CO2 are critical for catalyst activity and product selectivity. In situ diffuse reflectance infrared fourier transform spectroscopy (DRIFTS) experiments at 3 MPa confirm that both bi-HCOO* and m-HCOO* are the active intermediates for CO2 hydrogenation to methanol. The accumulation of m-HCOO* on the catalyst surface is the crucial step of CO2 hydrogenation to methanol.
中文翻译:

高压原位DRIFTS研究铜物种的多功能作用及在铜铈锆催化剂上CO 2加氢制甲醇的反应途径
制备了一系列含铜表面CuO和Cu-Ce-Zr固溶体两种铜的三元铜-铈-锆催化剂,并研究了CO 2加氢制甲醇的催化性能。与其他方法相比,在450°C下煅烧的铜-铈-锆催化剂(CCZ-450)更有利于改善表面CuO种类的性质并形成Cu-Ce-Zr固溶体。就甲醇选择性(T = 280°C,S CH3OH = 71.8%),周转频率(TOF CO2 = 13.4×10 -2 s -1)和活化能(E a)而言,最佳的催化行为 = 28.5 kJ / mol)是使用CCZ-450实现的。CCZ-450优异的催化性能归因于高度分散的表面CuO物种具有更高的H 2吸附能力,而CuO物种具有较高的铜表面积和较高的活性Bi / m-HCOO *中间体浓度,这是由于形成Cu-Ce- Zr固溶体。活性位点的分散度和表面积以及CO 2的活化能力对于催化剂活性和产物选择性都至关重要。在3 MPa下进行的原位漫反射红外傅里叶变换光谱(DRIFTS)实验证实,bi-HCOO *和m-HCOO *都是CO 2的活性中间体加氢成甲醇。m-HCOO *在催化剂表面的积累是CO 2加氢为甲醇的关键步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号