当前位置:
X-MOL 学术
›
J. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reductive alkylation of imines via asymmetric Cu-catalyzed addition of organozirconium reagents
Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2019-12-30 , DOI: 10.1016/j.jorganchem.2019.121099 Ivana Némethová , Denisa Vargová , Brigita Mudráková , Juraj Filo , Radovan Šebesta
中文翻译:

通过不对称铜催化的有机锆试剂的加成反应,使亚胺还原烷基化
更新日期:2019-12-30
Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2019-12-30 , DOI: 10.1016/j.jorganchem.2019.121099 Ivana Némethová , Denisa Vargová , Brigita Mudráková , Juraj Filo , Radovan Šebesta
|
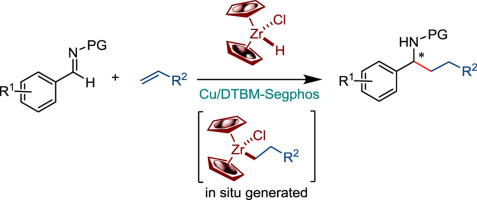
Chiral amines are important as medicines or agrochemicals. They are often assembled by nucleophilic addition to corresponding compounds featuring C=N bond. Pre-made organometallics are typical nucleophiles in this reaction. In this work, we describe asymmetric reductive alkylation of imines with alkenes. Hydrozirconation of these alkenes generated organozirconium species in situ. The transformation is catalyzed by Cu-Segphos complex and affords chiral amines in enantioselectivities up to 93% ee.
中文翻译:

通过不对称铜催化的有机锆试剂的加成反应,使亚胺还原烷基化
手性胺作为药物或农用化学品很重要。它们通常是通过亲核加成到具有C = N键的相应化合物中而组装的。预制的有机金属化合物是该反应中的典型亲核试剂。在这项工作中,我们描述了亚胺与烯烃的不对称还原烷基化。这些烯烃的加氢锆化可在原位生成有机锆物种。该转化由Cu-Segphos络合物催化,并提供对映体选择性高达93%ee的手性胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号