当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning Enzyme Activity for Nonaqueous Solvents: Engineering an Enantioselective "Michaelase" for Catalysis in High Concentrations of Ethanol.
ChemBioChem ( IF 2.6 ) Pub Date : 2020-02-18 , DOI: 10.1002/cbic.201900721 Chao Guo 1 , Lieuwe Biewenga 1 , Max Lubberink 1, 2 , Ronald van Merkerk 1 , Gerrit J Poelarends 1
ChemBioChem ( IF 2.6 ) Pub Date : 2020-02-18 , DOI: 10.1002/cbic.201900721 Chao Guo 1 , Lieuwe Biewenga 1 , Max Lubberink 1, 2 , Ronald van Merkerk 1 , Gerrit J Poelarends 1
Affiliation
|
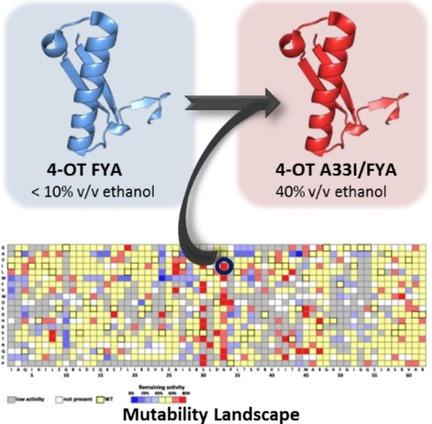
Enzymes have evolved to function under aqueous conditions and may not exhibit features essential for biocatalytic application, such as the ability to function in high concentrations of an organic solvent. Consequently, protein engineering is often required to tune an enzyme for catalysis in non-aqueous solvents. In this study, we have used a collection of nearly all single mutants of 4-oxalocrotonate tautomerase, which promiscuously catalyzes synthetically useful Michael-type additions of acetaldehyde to various nitroolefins, to investigate the effect of each mutation on the ability of this enzyme to retain its "Michaelase" activity in elevated concentrations of ethanol. Examination of this mutability landscape allowed the identification of two hotspot positions, Ser30 and Ala33, at which mutations are beneficial for catalysis in high ethanol concentrations. The "hotspot" position Ala33 was then randomized in a highly enantioselective, but ethanol-sensitive 4-OT variant (L8F/M45Y/F50A) to generate an improved enzyme variant (L8F/A33I/M45Y/F50A) that showed great ethanol stability and efficiently catalyzes the enantioselective addition of acetaldehyde to nitrostyrene in 40 % ethanol (permitting high substrate loading) to give the desired γ-nitroaldehyde product in excellent isolated yield (89 %) and enantiopurity (ee=98 %). The presented work demonstrates the power of mutability-landscape-guided enzyme engineering for efficient biocatalysis in non-aqueous solvents.
中文翻译:

调整非水溶剂的酶活性:设计对映选择性“ Michaelase”以在高浓度乙醇中进行催化。
酶已进化为在水性条件下起作用,并且可能未表现出生物催化应用必不可少的特征,例如在高浓度有机溶剂中起作用的能力。因此,通常需要蛋白质工程来调节酶以在非水溶剂中催化。在这项研究中,我们使用了几乎所有的4-草酰巴豆酸互变异构酶单个突变体,这些突变体混杂地催化了合成的有用的迈克尔型乙醛向各种硝基烯烃的加成反应,以研究每种突变对该酶保留能力的影响。在乙醇浓度升高时具有“ Michaelase”活性。通过检查这种可变性格局,可以确定两个热点位置:Ser30和Ala33,在高乙醇浓度下,这种突变有利于催化。然后将“热点”位置Ala33随机化为对映选择性高但对乙醇敏感的4-OT变体(L8F / M45Y / F50A),以产生改良的酶变体(L8F / A33I / M45Y / F50A),该变体显示出很好的乙醇稳定性和有效地催化在40%乙醇中将乙醛对映选择性地添加到硝基苯乙烯中(允许高的底物负载),从而以优异的分离收率(89%)和对映纯度(ee = 98%)得到所需的γ-硝基醛产物。提出的工作证明了可变性-景观引导的酶工程技术在非水溶剂中有效生物催化的能力。但对乙醇敏感的4-OT变体(L8F / M45Y / F50A)会产生改良的酶变体(L8F / A33I / M45Y / F50A),该变体显示出出色的乙醇稳定性,并有效催化40%乙醇中乙醛向硝基苯乙烯的对映选择性加成反应(允许较高的底物负载量)以优异的分离收率(89%)和对映体纯度(ee = 98%)得到所需的γ-硝基醛产物。提出的工作证明了可变性-景观引导酶工程技术在非水溶剂中有效生物催化的能力。但对乙醇敏感的4-OT变体(L8F / M45Y / F50A)会产生改良的酶变体(L8F / A33I / M45Y / F50A),该变体显示出出色的乙醇稳定性,并有效催化40%乙醇中乙醛向硝基苯乙烯的对映选择性加成反应(允许较高的底物负载量)以优异的分离收率(89%)和对映体纯度(ee = 98%)得到所需的γ-硝基醛产物。提出的工作证明了可变性-景观引导的酶工程技术在非水溶剂中有效生物催化的能力。
更新日期:2019-12-30
中文翻译:

调整非水溶剂的酶活性:设计对映选择性“ Michaelase”以在高浓度乙醇中进行催化。
酶已进化为在水性条件下起作用,并且可能未表现出生物催化应用必不可少的特征,例如在高浓度有机溶剂中起作用的能力。因此,通常需要蛋白质工程来调节酶以在非水溶剂中催化。在这项研究中,我们使用了几乎所有的4-草酰巴豆酸互变异构酶单个突变体,这些突变体混杂地催化了合成的有用的迈克尔型乙醛向各种硝基烯烃的加成反应,以研究每种突变对该酶保留能力的影响。在乙醇浓度升高时具有“ Michaelase”活性。通过检查这种可变性格局,可以确定两个热点位置:Ser30和Ala33,在高乙醇浓度下,这种突变有利于催化。然后将“热点”位置Ala33随机化为对映选择性高但对乙醇敏感的4-OT变体(L8F / M45Y / F50A),以产生改良的酶变体(L8F / A33I / M45Y / F50A),该变体显示出很好的乙醇稳定性和有效地催化在40%乙醇中将乙醛对映选择性地添加到硝基苯乙烯中(允许高的底物负载),从而以优异的分离收率(89%)和对映纯度(ee = 98%)得到所需的γ-硝基醛产物。提出的工作证明了可变性-景观引导的酶工程技术在非水溶剂中有效生物催化的能力。但对乙醇敏感的4-OT变体(L8F / M45Y / F50A)会产生改良的酶变体(L8F / A33I / M45Y / F50A),该变体显示出出色的乙醇稳定性,并有效催化40%乙醇中乙醛向硝基苯乙烯的对映选择性加成反应(允许较高的底物负载量)以优异的分离收率(89%)和对映体纯度(ee = 98%)得到所需的γ-硝基醛产物。提出的工作证明了可变性-景观引导酶工程技术在非水溶剂中有效生物催化的能力。但对乙醇敏感的4-OT变体(L8F / M45Y / F50A)会产生改良的酶变体(L8F / A33I / M45Y / F50A),该变体显示出出色的乙醇稳定性,并有效催化40%乙醇中乙醛向硝基苯乙烯的对映选择性加成反应(允许较高的底物负载量)以优异的分离收率(89%)和对映体纯度(ee = 98%)得到所需的γ-硝基醛产物。提出的工作证明了可变性-景观引导的酶工程技术在非水溶剂中有效生物催化的能力。









































 京公网安备 11010802027423号
京公网安备 11010802027423号