当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploiting a C-N Bond Forming Cytochrome P450 Monooxygenase for C-S Bond Formation.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-12-30 , DOI: 10.1002/anie.201916269 Iori Morita 1 , Takahiro Mori 1, 2 , Takaaki Mitsuhashi 1 , Shotaro Hoshino 1 , Yoshimasa Taniguchi 3 , Takashi Kikuchi 4 , Kei Nagae 5 , Norihiro Nasu 6 , Makoto Fujita 7, 8 , Tomohiko Ohwada 1 , Ikuro Abe 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-12-30 , DOI: 10.1002/anie.201916269 Iori Morita 1 , Takahiro Mori 1, 2 , Takaaki Mitsuhashi 1 , Shotaro Hoshino 1 , Yoshimasa Taniguchi 3 , Takashi Kikuchi 4 , Kei Nagae 5 , Norihiro Nasu 6 , Makoto Fujita 7, 8 , Tomohiko Ohwada 1 , Ikuro Abe 1, 2
Affiliation
|
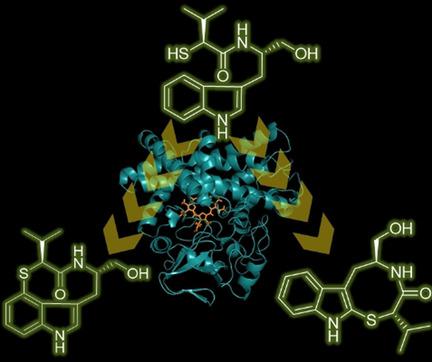
C-S bond formation reactions are widely distributed in the biosynthesis of biologically active molecules, and thus have received much attention over the past decades. Herein, we report intramolecular C-S bond formation by a P450 monooxygenase, TleB, which normally catalyzes a C-N bond formation in teleocidin biosynthesis. Based on the proposed reaction mechanism of TleB, a thiol-substituted substrate analogue was synthesized and tested in the enzyme reaction, which afforded the unprecedented sulfur-containing thio-indolactam V, in addition to an unusual indole-fused 6/5/8-tricyclic product whose structure was determined by the crystalline sponge method. Interestingly, conformational analysis revealed that the SOFA conformation is stable in thio-indolactam V, in sharp contrast to the major TWIST form in indolactam V, resulting in differences in their biological activities.
中文翻译:

利用CN键形成细胞色素P450单加氧酶进行CS键形成。
CS键形成反应广泛地分布在生物活性分子的生物合成中,因此在过去的几十年中受到了广泛的关注。在本文中,我们报道了通过P450单加氧酶TleB分子内CS键的形成,该酶通常会在Teleocidin生物合成中催化CN键的形成。根据拟议的TleB反应机理,合成并测试了巯基取代的底物类似物,并在酶反应中进行了测试,除具有不寻常的吲哚稠合的6/5 / 8-外,还提供了前所未有的含硫硫代吲哚内酰胺V。通过结晶海绵法确定其结构的三环产物。有趣的是,构象分析表明,SOFA构象在硫代吲哚内酰胺V中是稳定的,这与吲哚内酰胺V中的主要TWIST形式形成鲜明对比,
更新日期:2020-01-23
中文翻译:

利用CN键形成细胞色素P450单加氧酶进行CS键形成。
CS键形成反应广泛地分布在生物活性分子的生物合成中,因此在过去的几十年中受到了广泛的关注。在本文中,我们报道了通过P450单加氧酶TleB分子内CS键的形成,该酶通常会在Teleocidin生物合成中催化CN键的形成。根据拟议的TleB反应机理,合成并测试了巯基取代的底物类似物,并在酶反应中进行了测试,除具有不寻常的吲哚稠合的6/5 / 8-外,还提供了前所未有的含硫硫代吲哚内酰胺V。通过结晶海绵法确定其结构的三环产物。有趣的是,构象分析表明,SOFA构象在硫代吲哚内酰胺V中是稳定的,这与吲哚内酰胺V中的主要TWIST形式形成鲜明对比,











































 京公网安备 11010802027423号
京公网安备 11010802027423号