当前位置:
X-MOL 学术
›
Thermochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dilution enthalpies of LiBO2 and LiB5O8 aqueous solutions at 298.15 K and the application of ion-interaction model
Thermochimica Acta ( IF 3.1 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.tca.2019.178506 Fei Yuan , Long Li , Yafei Guo , Tianlong Deng
Thermochimica Acta ( IF 3.1 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.tca.2019.178506 Fei Yuan , Long Li , Yafei Guo , Tianlong Deng
|
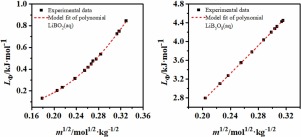
Abstract The dilution enthalpies of LiBO2 (aq) from 0.0398 to 0.7684 mol·kg−1 and LiB5O8 (aq) from 0.0521 to 0.8067 mol·kg−1 at 298.15 K and 101.325 kPa were investigated using the microcalorimeter BT 2.15. The apparent molar enthalpies on two lithium borates were obtained, and the diagrams of their apparent molar enthalpy against molality for LiBO2 (aq) and LiB5O8 (aq) were also plotted. Considering the constructers of BO2- and B5O8- in aqueous solution are existed as in [B(OH)4]- and [B5O6(OH)4]-, the Pitzer single-salt parameters ( β M X ( 0 ) L , β M X ( 1 ) L , β M X ( 2 ) L , C M X L ) for LiB(OH)4 and LiB5O6(OH)4 were obtained for the first time. A comparison between the calculated apparent molar enthalpies for LiBO2 (aq) and LiB5O8 (aq) based on Pitzer model and those determined experimentally agree well, which indicate that the Pitzer single parameters of LiB(OH)4 and LiB5O6(OH)4 obtained are reliable.
中文翻译:

LiBO2和LiB5O8水溶液在298.15 K下的稀释焓及离子相互作用模型的应用
摘要 使用微量热量计研究了在 298.15 K 和 101.325 kPa 条件下 LiBO2 (aq) 从 0.0398 到 0.7684 mol·kg-1 和 LiB5O8 (aq) 从 0.0521 到 0.8067 mol·kg-1 的稀释焓。获得了两种硼酸锂的表观摩尔焓,并且还绘制了它们的表观摩尔焓对 LiBO2 (aq) 和 LiB5O8 (aq) 摩尔浓度的图表。考虑到水溶液中 BO2- 和 B5O8- 的构建体存在于 [B(OH)4]- 和 [B5O6(OH)4]- 中,Pitzer 单盐参数 ( β MX ( 0 ) L , β MX ( 1 ) L , β MX ( 2 ) L , CMXL ) LiB(OH)4 和 LiB5O6(OH)4 首次得到。基于 Pitzer 模型计算出的 LiBO2 (aq) 和 LiB5O8 (aq) 的表观摩尔焓与实验确定的结果之间的比较非常吻合,
更新日期:2020-03-01
中文翻译:

LiBO2和LiB5O8水溶液在298.15 K下的稀释焓及离子相互作用模型的应用
摘要 使用微量热量计研究了在 298.15 K 和 101.325 kPa 条件下 LiBO2 (aq) 从 0.0398 到 0.7684 mol·kg-1 和 LiB5O8 (aq) 从 0.0521 到 0.8067 mol·kg-1 的稀释焓。获得了两种硼酸锂的表观摩尔焓,并且还绘制了它们的表观摩尔焓对 LiBO2 (aq) 和 LiB5O8 (aq) 摩尔浓度的图表。考虑到水溶液中 BO2- 和 B5O8- 的构建体存在于 [B(OH)4]- 和 [B5O6(OH)4]- 中,Pitzer 单盐参数 ( β MX ( 0 ) L , β MX ( 1 ) L , β MX ( 2 ) L , CMXL ) LiB(OH)4 和 LiB5O6(OH)4 首次得到。基于 Pitzer 模型计算出的 LiBO2 (aq) 和 LiB5O8 (aq) 的表观摩尔焓与实验确定的结果之间的比较非常吻合,









































 京公网安备 11010802027423号
京公网安备 11010802027423号