Chem ( IF 19.1 ) Pub Date : 2019-12-30 , DOI: 10.1016/j.chempr.2019.12.004 Yongquan Ning , Paramasivam Sivaguru , Giuseppe Zanoni , Edward A. Anderson , Xihe Bi
|
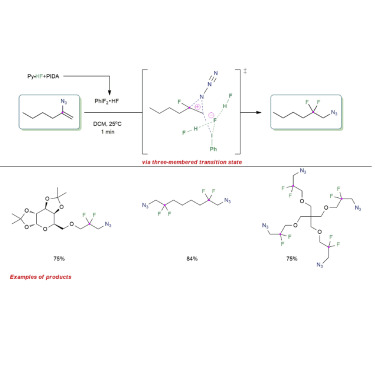
The development of azide migration reactions is a formidable challenge because of the potential competition of side processes driven by the release of molecular nitrogen. Here, we report a conceptually novel 1,2-azide migration in an unprecedented gem-difluorination of the readily available α-vinyl azides, a transformation that enables the synthesis of a range of novel β-difluorinated alkyl azides. The practicality of the method is demonstrated by broad substrate scope, excellent functional group compatibility, and high yields. The migrating group selectivity can be tuned through electronic effects, and DFT calculations suggest 1,2-azide migration occurs via a three-membered azacyclic transition state. By using routine protocols, the β-difluorinated alkyl azide products can be easily transformed to biologically relevant β-difluorinated amines—common structural motifs in pharmaceuticals, thus demonstrating the utility of these fluorinated organic azides for pharmaceutical synthesis as well as other synthetically useful derivatives.
中文翻译:

通过难于的1,2-叠氮化物迁移合成β-二氟烷基叠氮化物
叠氮化物迁移反应的发展是一个艰巨的挑战,因为由分子氮的释放驱动的副反应的潜在竞争。在这里,我们报告了前所未有的宝石中一种概念上新颖的1,2-叠氮化物迁移易得的α-乙烯基叠氮化物的-二氟化,这种转化使得能够合成一系列新型的β-二氟化烷基叠氮化物。该方法的实用性由广泛的底物范围,优异的官能团相容性和高产率证明。迁移基团的选择性可以通过电子效应来调节,DFT计算表明1,2-叠氮化物迁移是通过三元氮杂环过渡态发生的。通过使用常规规程,可以轻松地将β-二氟烷基叠氮化物产物转化为生物学上相关的β-二氟胺-药物中常见的结构图案,从而证明了这些氟化有机叠氮化物以及其他可用于合成的衍生物的实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号