Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-12-30 , DOI: 10.1016/j.tetlet.2019.151577 Junhui Jia , Juanjuan Wen
|
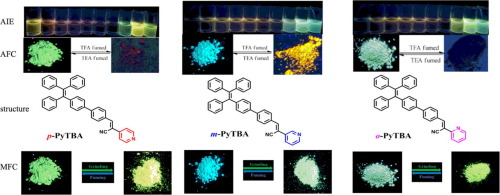
Three new positional isomers (Z)-2-(pyridin-2-yl)-3-(4'-(1,2,2-triphenylvinyl)-[1,1'-biphenyl]-4-yl)acrylonitrile (o-PyTBA), (Z)-2-(pyridin-3-yl)-3-(4'-(1,2,2-triphenylvinyl)-[1,1'-biphenyl]-4-yl)acrylonitrile (m-PyTBA) and (Z)-2-(pyridin-4-yl)-3-(4'-(1,2,2-triphenylvinyl)-[1,1'-biphenyl]-4-yl)acrylonitrile (p-PyTBA) incorporating tetraphenylethylene (TPE) as donor and pyridine unit and cyano-group as acceptor were designed and synthesized. The positional change exploited in these isomers influenced the acceptor strength and molecular packing. These three isomers displayed distinct solvatochromic behaviour and different aggregation induced emission (AIE) properties. The density functional theory (DFT) studies revealed good separation of the highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs) supporting the distinct intramolecular-charge transfer (ICT) transition. Moreover, the three isomers exhibited reversible mechanochromism in the order o-PyTBA (56 nm) > m-PyTBA (49 nm) > p-PyTBA (43 nm). The powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC) and field emission scanning electron microscopy (FESEM) studies indicated that the morphological conversion from crystalline to amorphous state should be responsible for the mechanochromism. Meanwhile, the extension in molecular conjugation caused by planarization of molecular conformation and subsequent planar intramolecular charge transfer (PICT) process by external force were responsible for the red-shifts in the fluorescence spectra. Interestingly, protonation-deprotonation of the pyridine moiety in o-PyTBA, m-PyTBA and p-PyTBA had a significant effect on the frontier molecular orbitals as well as very distinctive emission characteristics upon trifluoroacetic acid (TFA) and trietylamine (TEA) stimuli. The film of o-PyTBA, m-PyTBA and p-PyTBA achieved a low detection limit for trifluoroacetic acid which was estimated to be 13.2 ppm, 17 ppm and 7.2 ppm, respectively.
中文翻译:

DA四苯基乙烯官能化氰基吡啶异构体的多刺激响应荧光转换
三种新的位置异构体(Z)-2-(吡啶-2-基)-3-(4'-(1,2,2-三苯基乙烯基)-[1,1'-联苯] -4-基)丙烯腈(o -PyTBA),(Z)-2-(吡啶-3-基)-3-(4'-(1,2,2-三苯基乙烯基)-[1,1'-联苯] -4-基)丙烯腈(m -PyTBA)和(Z)-2-(吡啶-4-基)-3-(4'-(1,2,2-三苯基乙烯基)-[1,1'-联苯] -4-基)丙烯腈(p -PyTBA设计并合成了以四苯基乙烯(TPE)为供体,并以吡啶单元和氰基为受体的四苯基乙烯(TPE)。这些异构体中利用的位置变化影响受体强度和分子堆积。这三个异构体表现出不同的溶剂变色行为和不同的聚集诱导发射(AIE)特性。密度泛函理论(DFT)研究表明,最高占据分子轨道(HOMO)和最低未占据分子轨道(LUMO)的良好分离,支持了独特的分子内电荷转移(ICT)过渡。此外,这三种异构体表现出可逆的变色现象,顺序为o-PyTBA(56 nm)> m-PyTBA(49 nm)> p-PyTBA(43 nm)。粉末X射线衍射(PXRD),差示扫描量热法(DSC)和场发射扫描电子显微镜(FESEM)研究表明,从晶态到非晶态的形态转化应是导致机械致变色的原因。同时,由分子构象的平面化和随后的外力引起的平面分子内电荷转移(PICT)过程引起的分子共轭的扩展是荧光光谱中的红移的原因。有趣的是,o-PyTBA,m-PyTBA和p-PyTBA中吡啶部分的质子去质子化在三氟乙酸(TFA)和三乙胺(TEA)刺激下,对前沿分子轨道具有显着影响,并且具有非常独特的发射特性。o-PyTBA,m-PyTBA和p-PyTBA膜的三氟乙酸检出限很低,估计分别为13.2 ppm,17 ppm和7.2 ppm。











































 京公网安备 11010802027423号
京公网安备 11010802027423号