当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhanced Transformation of Emerging Contaminants by Permanganate in the Presence of Redox Mediators.
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2019-12-30 , DOI: 10.1021/acs.est.9b05711 Zhenyu Shi 1, 2 , Can Jin 3 , Ruopeng Bai 4 , Zhanqi Gao 2 , Jing Zhang 1 , Liang Zhu 1 , Zhiwei Zhao 1 , Timothy J Strathmann 5
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2019-12-30 , DOI: 10.1021/acs.est.9b05711 Zhenyu Shi 1, 2 , Can Jin 3 , Ruopeng Bai 4 , Zhanqi Gao 2 , Jing Zhang 1 , Liang Zhu 1 , Zhiwei Zhao 1 , Timothy J Strathmann 5
Affiliation
|
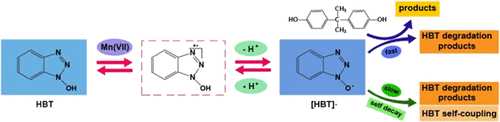
In this study, a permanganate/redox mediator system for enhanced transformation of a series of emerging contaminants was evaluated. The presence of various redox mediators (i.e., 1-hydroxybenzotriazole, N-hydroxyphthalimide, violuric acid, syringaldehyde, vanillin, 4-hydroxycoumarin, and p-coumaric acid) accelerated the degradation of bisphenol A (BPA) by Mn(VII). Since 1-hydroxybenzotriazole (HBT) exhibited the highest reactive ability, it was selected to further investigate the reaction mechanisms and quantify the effects of important reaction parameters on Mn(VII)/redox-mediator reactions with BPA and bisphenol AF (BPAF). Interestingly, not only HBT accelerated the degradation of BPA, but also BPA enhanced the decay of HBT. Evidence for the in situ formation of HBT· radicals as the active oxidant responsible for accelerated BPA and BPAF degradation was obtained by radical scavenging experiments and 31P NMR spin trapping techniques. The routes for HBT· radical formation involving Mn(VII) and the electron-transfer pathway from BPA/BPAF to HBT· radicals demonstrate that the Mn(VII)/HBT system was driven by the electron-transfer mechanism. Compared to Mn(VII) alone, the presence of HBT totally inhibited self-coupling of BPA and BPAF and promoted β-scission, hydroxylation, ring opening, and decarboxylation reactions. Moreover, Mn(VII)/HBT is also effective in real waters with the order of river water > wastewater treatment plant (WWTP) effluent > deionized water.
中文翻译:

在氧化还原介体存在下,高锰酸盐增强了对新兴污染物的转化。
在这项研究中,评估了用于增强一系列新兴污染物转化的高锰酸盐/氧化还原介体系统。各种氧化还原介体(即1-羟基苯并三唑,N-羟基邻苯二甲酰亚胺,紫尿酸,丁香醛,香兰素,4-羟基香豆素和对香豆酸)的存在加速了Mn(VII)对双酚A(BPA)的降解。由于1-羟基苯并三唑(HBT)表现出最高的反应能力,因此选择它来进一步研究反应机理并量化重要反应参数对与BPA和双酚AF(BPAF)进行的Mn(VII)/氧化还原介体反应的影响。有趣的是,不仅HBT加速了BPA的降解,而且BPA增强了HBT的降解。通过自由基清除实验和31P NMR自旋捕获技术获得了原位形成HBT·自由基作为负责加速BPA和BPAF降解的活性氧化剂的证据。涉及Mn(VII)的HBT·自由基形成的途径以及从BPA / BPAF到HBT·自由基的电子转移路径表明,Mn(VII)/ HBT体系是由电子转移机制驱动的。与单独的Mn(VII)相比,HBT的存在完全抑制了BPA和BPAF的自偶联,并促进了β断裂,羟基化,开环和脱羧反应。此外,Mn(VII)/ HBT在实际水中也有效,其顺序为河水>废水处理厂(WWTP)出水>去离子水。涉及Mn(VII)的HBT·自由基形成的途径以及从BPA / BPAF到HBT·自由基的电子转移路径表明,Mn(VII)/ HBT体系是由电子转移机制驱动的。与单独的Mn(VII)相比,HBT的存在完全抑制了BPA和BPAF的自偶联,并促进了β断裂,羟基化,开环和脱羧反应。此外,Mn(VII)/ HBT在实际水中也有效,其顺序为河水>废水处理厂(WWTP)出水>去离子水。涉及Mn(VII)的HBT·自由基形成的途径以及从BPA / BPAF到HBT·自由基的电子转移路径表明,Mn(VII)/ HBT体系是由电子转移机制驱动的。与单独的Mn(VII)相比,HBT的存在完全抑制了BPA和BPAF的自偶联,并促进了β断裂,羟基化,开环和脱羧反应。此外,Mn(VII)/ HBT在实际水中也有效,其顺序为河水>废水处理厂(WWTP)出水>去离子水。羟基化,开环和脱羧反应。此外,Mn(VII)/ HBT在实际水中也有效,其顺序为河水>废水处理厂(WWTP)出水>去离子水。羟基化,开环和脱羧反应。此外,Mn(VII)/ HBT在实际水中也有效,其顺序为河水>废水处理厂(WWTP)出水>去离子水。
更新日期:2020-01-14
中文翻译:

在氧化还原介体存在下,高锰酸盐增强了对新兴污染物的转化。
在这项研究中,评估了用于增强一系列新兴污染物转化的高锰酸盐/氧化还原介体系统。各种氧化还原介体(即1-羟基苯并三唑,N-羟基邻苯二甲酰亚胺,紫尿酸,丁香醛,香兰素,4-羟基香豆素和对香豆酸)的存在加速了Mn(VII)对双酚A(BPA)的降解。由于1-羟基苯并三唑(HBT)表现出最高的反应能力,因此选择它来进一步研究反应机理并量化重要反应参数对与BPA和双酚AF(BPAF)进行的Mn(VII)/氧化还原介体反应的影响。有趣的是,不仅HBT加速了BPA的降解,而且BPA增强了HBT的降解。通过自由基清除实验和31P NMR自旋捕获技术获得了原位形成HBT·自由基作为负责加速BPA和BPAF降解的活性氧化剂的证据。涉及Mn(VII)的HBT·自由基形成的途径以及从BPA / BPAF到HBT·自由基的电子转移路径表明,Mn(VII)/ HBT体系是由电子转移机制驱动的。与单独的Mn(VII)相比,HBT的存在完全抑制了BPA和BPAF的自偶联,并促进了β断裂,羟基化,开环和脱羧反应。此外,Mn(VII)/ HBT在实际水中也有效,其顺序为河水>废水处理厂(WWTP)出水>去离子水。涉及Mn(VII)的HBT·自由基形成的途径以及从BPA / BPAF到HBT·自由基的电子转移路径表明,Mn(VII)/ HBT体系是由电子转移机制驱动的。与单独的Mn(VII)相比,HBT的存在完全抑制了BPA和BPAF的自偶联,并促进了β断裂,羟基化,开环和脱羧反应。此外,Mn(VII)/ HBT在实际水中也有效,其顺序为河水>废水处理厂(WWTP)出水>去离子水。涉及Mn(VII)的HBT·自由基形成的途径以及从BPA / BPAF到HBT·自由基的电子转移路径表明,Mn(VII)/ HBT体系是由电子转移机制驱动的。与单独的Mn(VII)相比,HBT的存在完全抑制了BPA和BPAF的自偶联,并促进了β断裂,羟基化,开环和脱羧反应。此外,Mn(VII)/ HBT在实际水中也有效,其顺序为河水>废水处理厂(WWTP)出水>去离子水。羟基化,开环和脱羧反应。此外,Mn(VII)/ HBT在实际水中也有效,其顺序为河水>废水处理厂(WWTP)出水>去离子水。羟基化,开环和脱羧反应。此外,Mn(VII)/ HBT在实际水中也有效,其顺序为河水>废水处理厂(WWTP)出水>去离子水。



























 京公网安备 11010802027423号
京公网安备 11010802027423号